del Pino / Pelaz
Research themes
Functional Nanoestructured Materials: Tools for Biomedical Applications
Main researcher(s)
Group members
| Pérez Potti, André |
Junior Scientist |
|
| Alameda Felgueiras, María Teresa |
Postdoctoral Researcher |
|
| Ceballos Guzmán, Juan Manuel |
Postdoctoral Researcher |
|
| Cruz Ortiz, Andrés Felipe |
Postdoctoral Researcher |
|
| Da Silva Álvarez, Sabela |
Postdoctoral Researcher |
|
| Fazal, Sajid |
Postdoctoral Researcher |
|
| Padín González, Esperanza |
Postdoctoral Researcher |
|
| Velasco Rodríguez, Brenda |
Postdoctoral Researcher |
|
| Zampini, Giulia |
Postdoctoral Researcher |
|
| Aguilera Llavero, María |
PhD Candidate |
|
| Cabello Huerta, Mar |
PhD Candidate |
|
| Durán Bravo, Álvaro |
PhD Candidate |
|
| Fernández Iglesias, Antía |
PhD Candidate |
|
| Fernández Vega, Javier |
PhD Candidate |
|
| Funes Hernando, Samuel |
PhD Candidate |
|
| Gkoutzamanis, Antonios |
PhD Candidate |
|
| Liodakis, Nikolaos |
PhD Candidate |
|
| Sánchez Martínez, Laura |
PhD Candidate |
|
| Zerrouk, Fatima |
PhD Candidate |
Research
Welcome to the research site of the BioNanoTools laboratory at CIQUS.
Our research is focused on the development of multifunctional composite materials for applications in biology and medicine. We design, synthesize and customize nano- and micro-materials with applications in therapy, diagnostics and imaging.
We are working at the interface of materials science, biology and medicine with the goal of producing next generation "smart" materials, having enhanced multifunctional capabilities.
We are interested in exploring radically new solutions to scientific challenges in biology and medicine, as well as in basic research in materials with interesting new properties (e.g., nanoMOFs, nanoperovskites, etc.).
See more details in the description of our research lines.
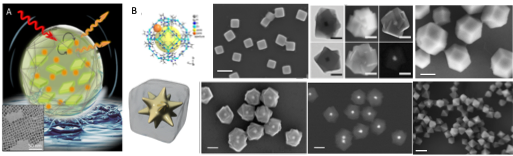
COLLOIDAL NANOPARTICLES (NPs)
We develop, optimize and adapt bottom-up synthetic routes for the production of NPs made of different inorganic materials, such as QDs, plasmonic NPs (Au, Ag, Cu), and magnetic NPs (iron oxides, FePt, Mn/Co/Zn Fe-substituted ferrites). We can easily adjust the size, shape and coatings, which allow us to produce colloidally stable, robust NPs for a variety of bio-applications.
Currently, we also work on the production of nanocomposites based on inorganic NPs and MOF (metal-organic frameworks), as well as other exciting, new materials, such as NPs made of perovskite semiconductors.
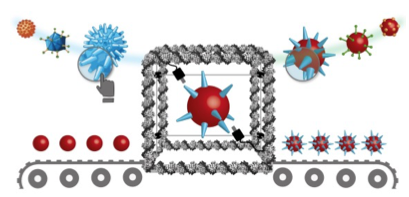
DNA-ORIGAMIS AS PRINTERS TO MODIFY THE SURFACE OF COLLOIDAL NANOMATERIALS
DNA strands can be folded on-demand to produce 3D structures with almost atomic precision (DNA-origamis). Our goal is to adapt this technology to produce molecular nanoprinters capable to imprint 3D ligand patters on the surface of nanomaterials, aiming mimicking, for example, the protein patters that viruses express naturally in their capsids. The outcomes of this project will help us to understand how the number and the special disposition of the ligands determine not only how nanomaterials interact with cells but also their biological fate. All these will lead to the design of more efficient nanomedicines.
NANOHEATING & NANOREACTORS
Interaction of light or AMF with inorganic NPs is orders of magnitude stronger than with organic molecules, in particular due to their larger absorption cross-sections. Therefore inorganic NPs offer a convenient platform for remotely controlled heating via light or AMF.
We develop very efficient multifunctional nanoheaters based on plasmonic or magnetic NPs for a variety of bioapplications, such as photothermal therapy, magnetic hyperthermia, optoacoustic imaging and thermal biosensing.
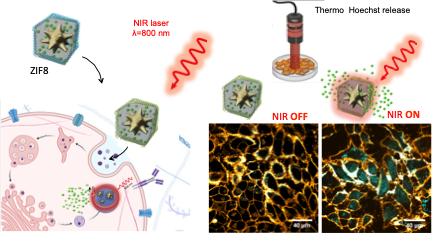
STIMULI-RESPONSIVE DRUG CARRIERS
We develop multifunctional, composite carriers based on layer-by-layer polymer capsules and plasmonic/magnetic NPs, which upon interaction with external stimuli, such as light or alternating magnetic fields (AMF), release their content into the cytosol of cells.
We can encapsulate a variety of macromolecules of bio-medical relevance, including siRNA, miRNA, proteins, small hydrophobic drugs, etc.
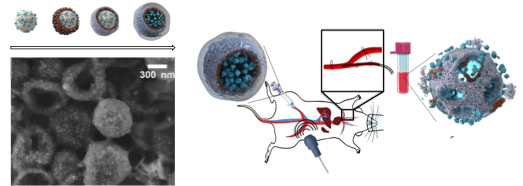
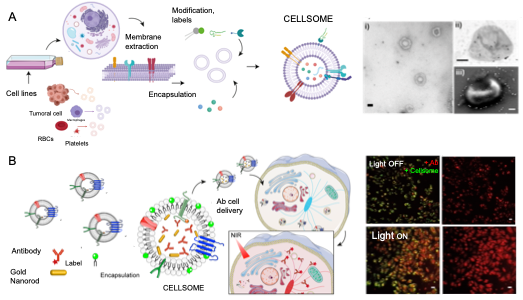
SMART BIOMIMETIC NANOSYSTEMS
We seek for developing the next generation of “smart” biomimetic materials, mimicking natural cellular structures (such as cell membranes) with the goal of producing stimuli-responsive drug delivery nanoformulations. Translating cell membrane features and thus, being capable of implementing their surface properties on nanomedicines, offer exciting opportunities to fabricate next generation biomimetic nanoformulations with enhanced pharmacokinetic and tissue-specific targeting capabilities. Such biomimetic interfaces have emerged to overcome the main drawbacks inherent to synthetic nanomaterials. Cell membrane-derived nanostructures have proven to be the future of bioinspired synthetic nanocarriers for vaccines and drug-delivery systems.
We design novel biological nanostructures (biomimetic nanosystems), which combine cell membrane components and inorganic NPs to create functional nano bio-inorganic assemblies with physical (e.g., inorganic NPs) and biomimetic capabilities. We aim to develop a novel class of a versatile stimuli response drug delivery system for potential application in the treatment of diseases with specificity, by selecting the specific cargo and a biomimetic coating capable of achieving in vivo targeting and evading the host defense system.
NANOBIOINTERACTIONS
We are interested in characterizing the interaction of “our” materials with biologically relevant entities, such as proteins (protein corona), cells and animals.
Therefore, we use and develop methods (e.g., colloidal stability, nanotoxicology, internalization, etc.) to assess the impact of our materials in living entities, as well as to characterize what happens to “our materials” in biologically relevant environments (e.g., cell cultures, animals, etc.).
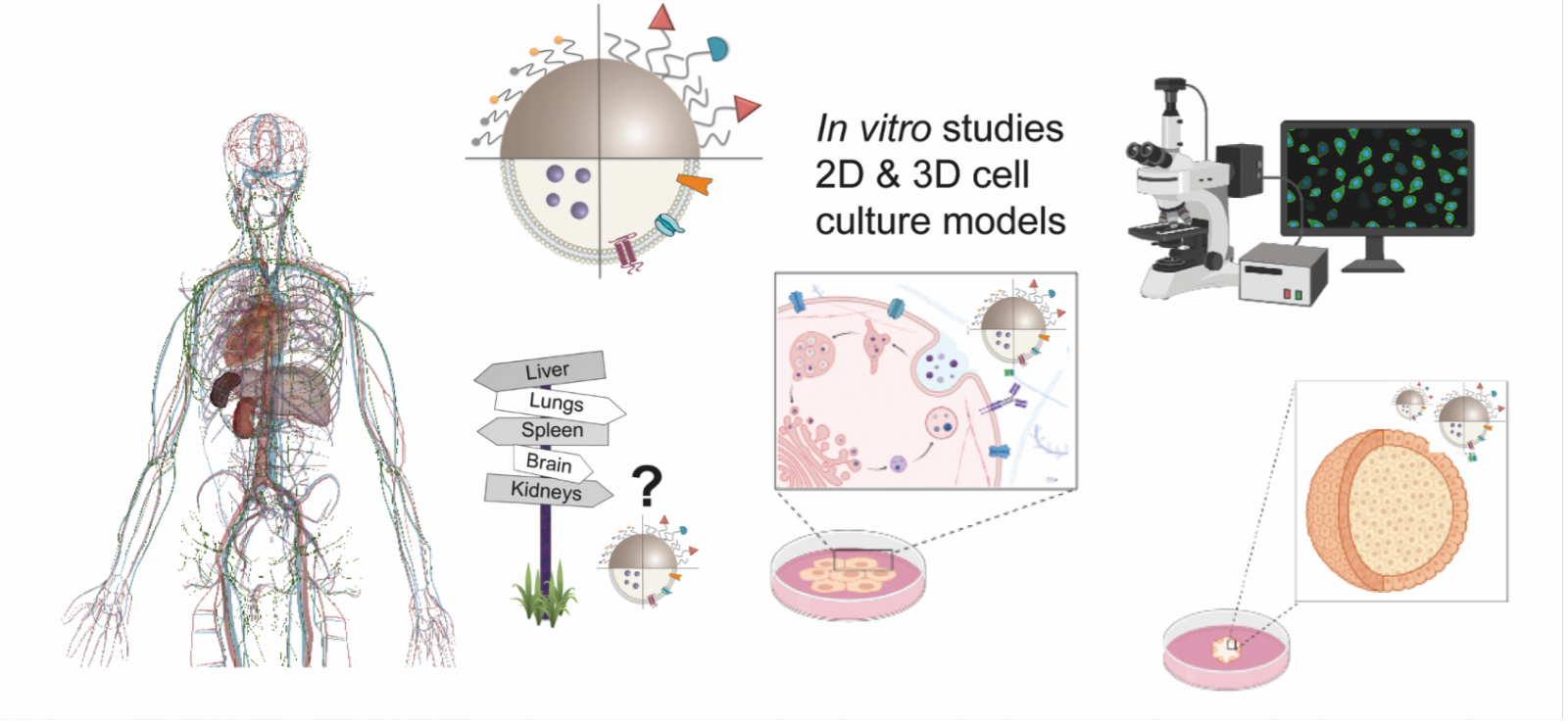
Fig. 6: Different scenarios and biological barriers in which NPs are present once they are injected in vivo. ACS Nano, 2017, 11 (3), pp 2397–2402.





