Sardina
Research themes
Reactivity and Catalysis
Group members
| Nogueira Vázquez, Carlos |
Technical staff |
|
| Trillo Mouzo, Alicia |
Administrative staff |
Research
Development of new “GREEN” C-C bond forming and bond breaking reactions
Our group is developing a line of research aimed at obtaining organic substances with high added value from commodity chemicals (affordable commercial products available in ton scale at very low prices), in a few synthetic steps and using reagents that do not pose environmental risks or economic disadvantages.
Our main synthetic objectives are substances that possess molecular frameworks or stereochemical features difficult to prepare by conventional synthetic procedures, such as those shown in the following figures.

We are developing new C-C bond-forming reactions by harvesting the potential of multiply charged synthetic intermediates (generated by reductive processes mediated by lithium or sodium metals or tin nucleophiles), and new ring opening processes based on stereoselective electrocyclic reactions of anion radicals.
Cation-Π interactions in small organic molecules. Physico-chemical studies and applications in synthesis
The cation-Π interaction is mainly an electrostatic interaction between positively charged species (a cation) and the electrons that are part of one or more Π bonds. Thanks to this type of interaction it has been possible to explain a number of rearrangements, and instances of catalytic reactions and anquimeric assistance within a wide range of contexts.
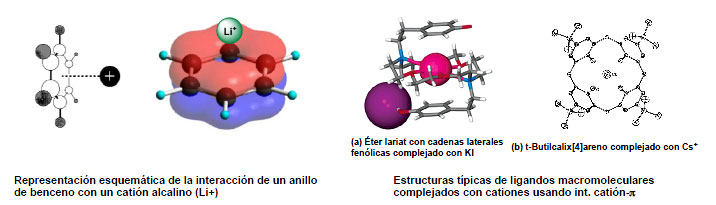
It has been determined by gas-phase studies, theoretical and experimental, that this type of interaction is one of the stronger non-covalent bonding forces, whose intensity is comparable to that of the ion-dipole interaction (see figure).
The effects of cation complexation due to noncovalent interactions originated by unsaturated systems play a role in most areas of molecular biology and modern chemistry (from structure and aggregation to reactivity and selectivity). Being able to understanding this interaction is essential for designing new drugs, since such interactions are responsible for enzyme recognition, besides being one of the causes of protein folding and the key to understanding the mechanism of ion channels operation.
This type of interactions has been rarely used, especially in the context of the complexation of alkali metals, of great importance in biochemistry (two representative examples are shown on the figure).
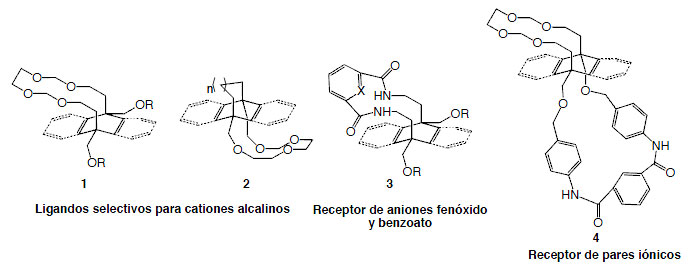
The first objective of this project is the design, synthesis and evaluation of macromolecular ligands for alkali cations, of the crown ether type, that incorporate aromatic rings that can induce selective cation-π interactions with Li +, Na +, K +, Rb + and Cs +, the goal being the generation catalytic systems innovative architectures, based on the Lewis acid properties of the cations in highly lipophilic (compounds types 1 and 2).
The second objective is the preparation of amide-type systems (compounds type 3) and their study as aromatic anion receptors, and ditopic receptors that can complex a cation and an anion at the same time (compounds of type 4) and its study as selective transporters.
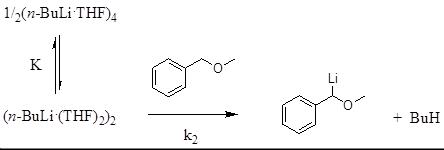
Org. React. 2013, 15, 4090-4093.
Physical organic chemical studies of organolithium reactivity
In collaboration with the research group of Prof. Luis García-Río, we are applying kinetic analyses and NMR spectroscopy to unravel the role of precomplexation of RLi species to basic centres in organic molecules in the reactivity of organolithium reagents as bases and nucleophiles.