Rodríguez / Ríos
Research themes
Biophysical Chemistry, Photophysics and Spectroscopy
Research
Our research aims to understand the dynamics of molecular processes induced by light absorption. We study the rates and mechanisms of photophysical and photochemical processes, especially photoinduced processes of proton transfer, electron transfer and energy transfer. In addition, we investigate the light response of molecular systems to obtain information on the structure and properties of molecular materials and biological systems.
The main technique we use is time-resolved fluorescence spectroscopy, which allows us to measure ultrafast photoinduced processes that take place at different time scales, down to picoseconds.
The aims of our research are:
- To understand the dynamics of photoinduced molecular processes, especially proton transfer, electron transfer and energy transfer.
- To characterize the dynamics of photoinduced processes in molecular materials and biological systems;
- To design new fluorescent probes for the structural and dynamic study of chemical and biological systems;
- To develop new molecular materials with defined photophysical properties.
Dynamics and mechanism of photoinduced proton- and electron-transfer processes in molecular materials and biological systems
One of our main research interests is the study of the dynamics and mechanism of proton transfer processes. This field of research is driven by basic questions in the area of acid-base chemistry and by puzzling mechanistic questions in the areas of proton transport across biological membranes or across the proton-exchange membranes of fuel cells, considered as the main "green" alternatives to current combustion engines.
The study of photoinduced proton-transfer reactions is a powerful approach to investigate the dynamics of proton-transfer processes. Molecules bearing acid or basic groups may experience drastic changes in their acid-base properties upon electronic excitation. For these molecules, absorption of light triggers proton transfer reactions whose dynamics can be studied on ultrafast time scales with spectroscopic techniques. By measuring transient absorption and time-resolved fluorescence, we were able to show that the intrinsic rate of proton transfer from a strong acid to the solvent is controlled by solvation (J. Am. Chem. Soc. 2007), and that the process takes place in steps (J. Phys. Chem. B 2013 and J. Phys. Chem. Lett. 2014).
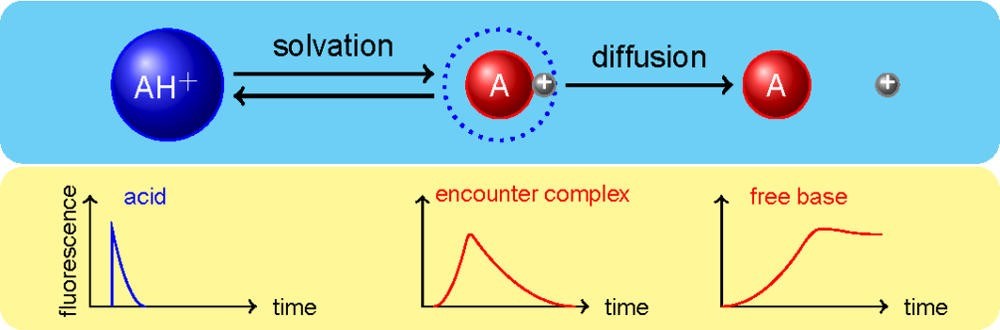
Dissociation of a strong acid in alcohols.
Excited-state proton-transfer reactions are often coupled to electron-transfer processes, as electronic excitation brings about a strong increase of molecular electron donor and acceptor abilities. Besides, the coupling of electron and proton transfer catalyses redox processes by avoiding high-energy intermediates.
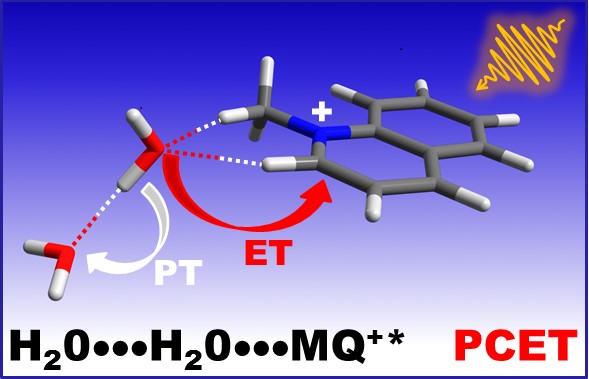
In a recent work (Phys. Chem. Chem. Phys. 2018) we were able to show that water (or alcohol) dimers play a fundamental role in the fluorescence quenching of N-methylquinolinium. Our results are consistent with the existence of a concerted photoinduced proton-coupled electron transfer (PCET) involving an intermediate complex of the excited quinolinium with a H-bonded molecular pair of the hydroxy compounds. In these pairs, a water or alcohol molecule is able to donate an electron to the photoexcited quinolinium cation and a proton to the second H-bonded hydroxy molecule, showing an enhanced reducing power in comparison with the isolated molecule.
Our results may be relevant to the study of renewable energy sources, solar water splitting and solar fuel production, and contribute to understanding the puzzling photorelaxation and electron transfer mechanisms of biomolecules, where water molecules have been shown to play a fundamental role.