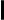|
|
|
Molecular pharmacology of G-protein coupled receptors (GPCRs) |
|
G-protein coupled receptors (GPCRs) are proteins present in the plasma membrane, which are activated by extracellular ligands of diverse nature (neurotransmitters, hormones, small chemical agents) that transmit signals to the interior of the cell, thereby producing cellular events that determine a large number of biological processes. The functionality and expression of these receptors are altered in many human pathologies, such as psychiatric and cardiovascular diseases, endocrine disorders and cancer. In fact, GPCRs are the targets of more than 70% of the drugs currently used. 
This line of research centres on the study of the molecular mechanisms that control the expression and functionality of GPCRs that are relevant in human pathologies such as schizophrenia. These mechanisms operate at the genetic level (polymorphisms in the genes that code for these receptors, epigenetic control of expression of these genes), the transcription level (stability of RNA messenger) or the protein receptor level (post-translational modifications with associated variations in desensitization, internalization or degradation of the receptor, alterations in the conformation of the protein receptor that modify its function or interaction with other receptors on the cell surface, with other components of the plasma membrane or with other translational proteins, effectors or intracellular regulators). We are interested in approaching this study in the context of a complex disease (schizophrenia), and have available genetic, transcriptional and conformational information from both human samples and from simple, readily available and easy-to-handle cellular models. Some of the projects already underway, as well as future objectives, include:
Fig. 1: Principle of an experiment for the detection of conformational changes 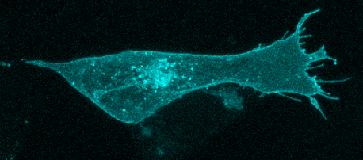 Fig. 2: Expression of serotonin receptor 5-HT2A tagged to a CFP fluorescent protein in the human neuroblastoma SH-SY5Y cell line. Basal conditions (no agonist).
Main researcher: Marian Castro Pérez, Ph. D. |
|
Biofarma Group | CIMUS Building, Avenida Barcelona S/N, 15782 Santiago de Compostela, A Coruña | Tel: 881 815 459 | Email: biofarma.group@usc.es| |