Guitián / Pérez / Peña
Research themes
Main researcher(s)
Emeritus University Professor(s)
Group members
| Guitián Rivera, Enrique | ||
| Bello García, Jesús |
Postdoctoral Researcher |
|
| Castro Fernández, Silvia |
Postdoctoral Researcher |
|
| Mateo de Doni, Luis Manuel |
Postdoctoral Researcher |
|
| Mora Fuentes, Juan Pedro |
Postdoctoral Researcher |
|
| Pozo Míguez, Iago |
Postdoctoral Researcher |
|
| Rodrigues Vilares Cabral Monteiro, Ana Rita |
Postdoctoral Researcher |
|
| Vilas Varela, Manuel |
Postdoctoral Researcher |
|
| Besteiro Saez, Javier |
PhD Candidate |
|
| Gómez Rodrigo, Lucía |
PhD Candidate |
|
| Janeiro Rodríguez, Jesús |
PhD Candidate |
|
| Limeres Vázquez, Carlos |
PhD Candidate |
|
| Luaces Calvín, Antón |
PhD Candidate |
|
| Malavé Fernández, María Valentina |
PhD Candidate |
|
| Martínez Castrillón, Adrián |
PhD Candidate |
|
| Rey Bello, Nicolás |
PhD Candidate |
|
| Villar Castro, Daniel |
PhD Candidate |
|
| Cobas Martínez, Agustín |
Inv. collaborator |
|
| Reif López, Rubén |
Scientific Management Technical Staff |
|
| Fernández Aguiño, Carmen |
Lab Technical Staff |
Research
We are a research group interested on using organic chemistry to solve long-standing scientific challenges, mainly in the fields of aromatic chemistry and carbon-based materials. We are specialized in the development of new synthetic methodologies based on aryne chemistry, the preparation of large aromatic molecules and functionalized materials, the development of bottom-up approaches to graphene materials and the combination of solution chemistry with surface synthesis. In the last 10 years we have published more than 70 research articles including 3 in Science, 2 in Nature Chemistry, 2 in Nature Commun., 6 in Angewandte Chemie, 3 in J. Am. Chem. Soc. or 8 in ACS Nano.
Our research group (COMMO, GI-1565), formed by ca. 20 people coordinated by Enrique Guitián, has been recently recognized as Grupo de Referencia Competitiva of the Galician University System. In terms of on-going projects and funding we have been awarded with two projects from the Agencia Estatal de Investigación (2020-23; CTQ, aryne-based “molecular Lego” and MAT, solution synthesis for functional molecular nanosystems), one EU-funded FET Open (2019-23, SPRING: Spin research in graphene) and one EU-funded FLAG-ERA (2020-23, Legochip: nanoporous graphene integration in biosensors).

Very recently Diego Peña has been awarded with an ERC-Synergy Grant (2021-27, MolDAM: Single Molecular Devices by Atomic Manipulation) together with Leo Gross (IBM Research, Zurich) and Jascha Repp (University Regensburg). MolDAM aims at building and controlling individual molecules through their manipulation with sophisticated microscopes.

At present we work in two main research topics:
1. Synthetic methodology based on aryne chemistry. Coordinated by Dolores Pérez.
A main interest in our group is the development of new synthetic methods based in the chemistry of short-lived aryne species, and the application of these methods to the synthesis and study of structurally fascinating large aromatic molecules and novel π-functional material. Our strategies involve both the development of novel aryne building blocks, and also the combination of the classical chemistry of arynes (e.g. [4+2] cycloadditions) with new reactions discovered in our laboratories. One of our main contributions to the field has been the development of the metal-catalyzed [2+2+2] cycloaddition reactions of arynes, which have become the standard procedure for the synthesis of three-branched polycyclic aromatic compounds, such as starphenes:
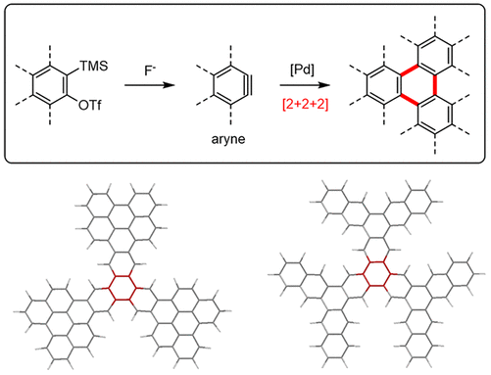
Acc. Chem. Res. 2019, 52, 2472-2481
With regard to the development of new aryne building blocks, we work on the design and synthesis of suitable precursors of new polyarynes, such as 2,6,10-triphenylenotriyne, from which the selective generation and trapping of each individual triple bond allow different transformations in each branch and, thus, the access to diverse triphenylene-based extended PAHs. We have also synthesized new complex aryne building blocks such as a fullerobenzyne, which has been used for the preparation of graphene-C60 nanoconjugates (in collaboration with N. Martín, UCM).
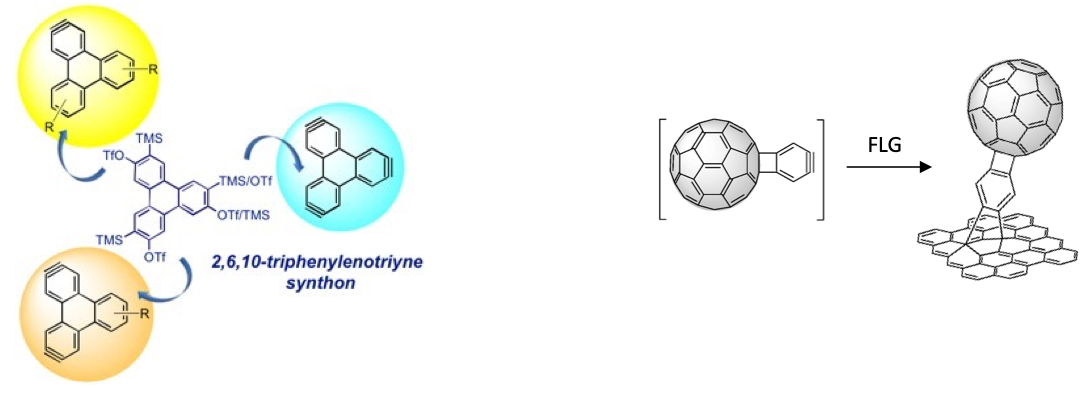
2,6,10-Triphenylenotriyne: a star-shaped trisaryne A C60-aryne building-block: synthesis of a hybrid all-carbon nanostructure
Chem. Commun. 2020, 56, 12853-12856 Chem. Commun. 2016, 52, 6677-6680
New synthetic strategies based on bisaryne cycloadditions are particularly useful for the synthesis of linear and cyclic acenes. A tetraepoxycyclacene obtained by these strategies, has been partially deoxygenated by STM-based atom manipulation, and the corresponding molecular belts visualized by AFM with CO-functionalized tips (in collaboration with L. Gross, IBM Research).
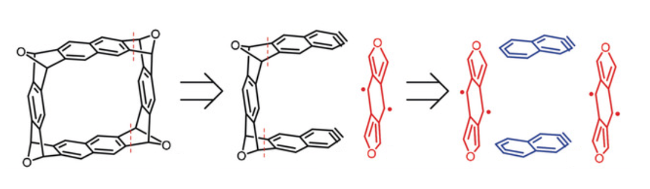
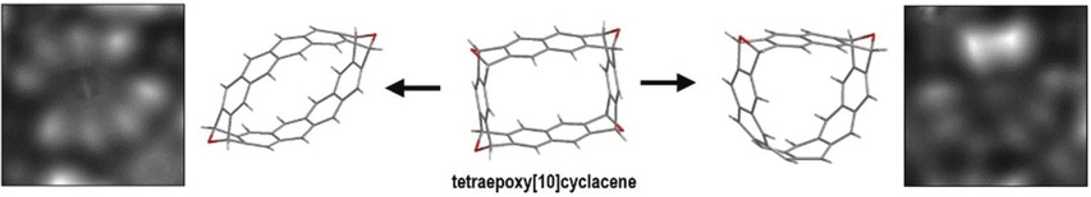
Exploring a route to cyclic acenes by on-surface synthesis
Angew. Chem. Int. Ed. 2019, 58, 9038-9042
We have also applied novel aryne chemistry to solve fundamental chemical questions, such as the long-standing debate over the aromaticity of kekulene. The complementary use of synthetic methodologies based on aryne cycloadditions, computational studies, and state-of-the art scanning probe microscopies shed light on the molecular and electronic structure of this iconic PAH (in collaboration with L. Gross, IBM Research and M. Melle-Franco, Univ. Aveiro).
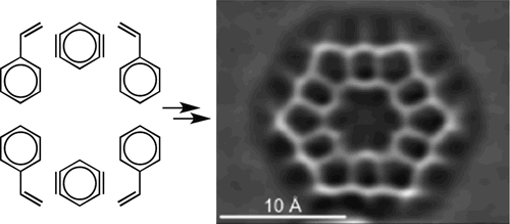
Revisiting Kekulene: Synthesis and Single-Molecule Imaging
J. Am. Chem. Soc. 2019, 141, 15488-15493
2. Bottom-up approaches to well-defined nanosized graphenes. Coordinated by Diego Peña.
In recent years, we have introduced new synthetic methods to prepare well-defined nanographenes by solution chemistry. We work in close collaboration with groups specialized in Scanning Tunneling Microscopy (STM) and Atomic Force Microscopy (AFM), in order to study these graphene materials. We also combine solution chemistry with surface synthesis to obtain “à la carte” designed graphenes, including graphene nanoribbons (GNRs) and nanoporous graphenes (NPGs). Some selected, recent results of our work in this topic are shown below.
For example, we built a triporous nanographene formed by 102 sp2 carbon atoms by combining a Pd-catalyzed aryne cyclotrimerization in solution with on-surface Au-promoted cyclodehydrogenations. Single molecules of this triporous nanographene were characterized by high resolution AFM (in collaboration with S. Godlewski, Jagiellonian University, Krakow)
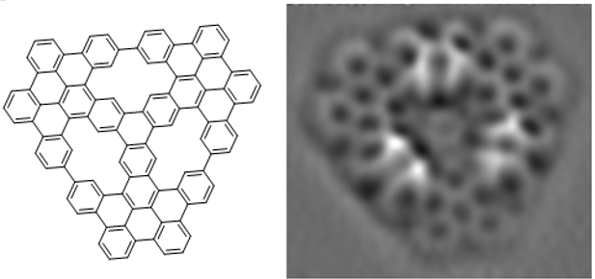
Synthesis and reactivity of a trigonal porous nanographene on a gold surface
Chem. Sci. 2019, 10, 10143-10148
The careful design of the molecular precursor is the key issue to obtain the desired graphene derivative. In collaboration with the groups of Nacho Pascual (nanoGUNE) and Dimas de Oteyza (CFM) we work on the preparation of precursors for graphene nanoribbons (GNRs). For example, we demonstrated that it is possible to transfer the axial chirality of the enantiomerically enriched graphene precursor to the planar chirality of the GNR adsorbate, which were analysed by high resolution STM.
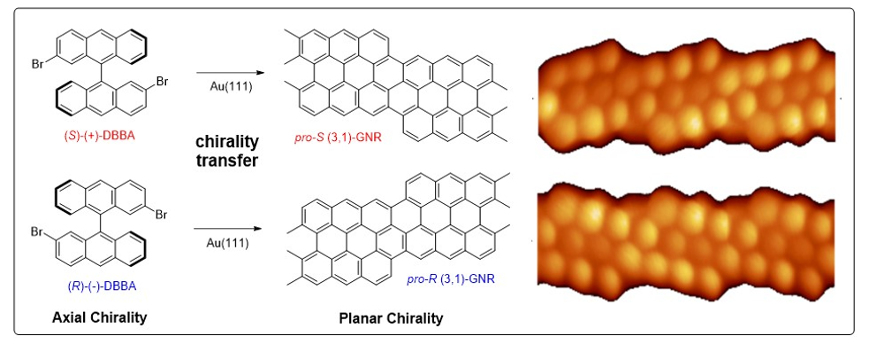
Transferring axial molecular chirality through a sequence of on-surface reactions
Chem. Sci. 2020, 11, 5441-5446
The molecular design can be modified to obtain other well-defined graphene materials. In collaboration with the groups of Aitor Mugarza (ICN2) and Aran García-Lekue (DIPC) we prepared nanoporous graphenes (NPGs) by a sequence of on-surface reactions on gold. The introduction of nanosized pores makes NPG permeable and semiconductor with potential applications as molecular filters and sensors. Dissemination movie: Nanoporous graphene for smart filters and sensors.
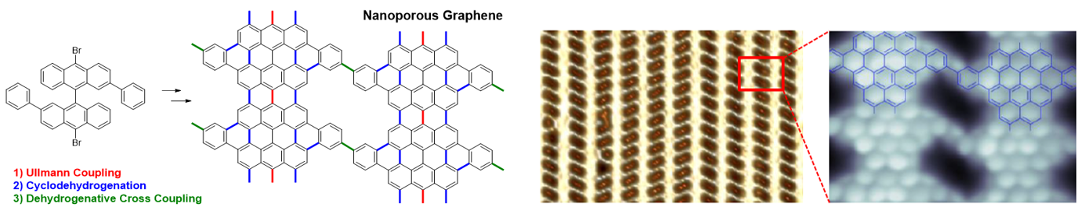
Bottom-up synthesis of multifunctional nanoporous graphene
Science 2018, 360, 199-203
Some of our bottom-up approaches rely on chemical transformations developed on different surfaces. In our long-standing collaboration with the group of Leo Gross (IBM, Zurich) we aim to control and visualize the reactivity of single molecules. For example, recently we demonstrated the coupling of terminal alkynes in single molecules by atom manipulation. This project is closely related to the ERC-Synergy Grant MolDAM which aims to build single molecules atom by atom and to take control over the way molecules behave and react.
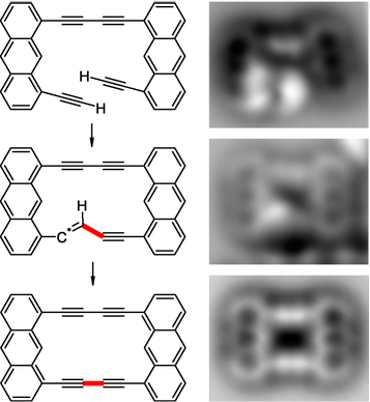
Intramolecular coupling of terminal alkynes by atom manipulation
Angew. Chem. Int. Ed. 2020, 59, 22989-22993





