Quiñoá / Fernández-Megía / Estévez
Research themes
Main researcher(s)
Emeritus University Professor(s)
Group members
| Estévez Cabanas, Ramón J. | ||
| Riguera Vega, Ricardo Jesús | ||
| Correa Chinea, Juan Francisco |
Postdoctoral Researcher |
|
| Hamidkhan Pathan, Shaheen |
Postdoctoral Researcher |
|
| Delgado González, Bruno |
PhD Candidate |
|
| Fernández Míguez, Manuel |
PhD Candidate |
|
| García Abuín, Lucas |
PhD Candidate |
|
| Jiménez López, Celia |
PhD Candidate |
|
| Landín González, Helena |
PhD Candidate |
|
| Lorenzo Fojón, Carla |
PhD Candidate |
|
| Freire Iribarne, Félix |
Inv. collaborator |
|
| Seco Castro, José Manuel |
Inv. collaborator |
Research
NANOSTRUCTURES FOR BIOMEDICAL APPLICATIONS
Supervised by Prof. Ricardo Riguera and Prof. Eduardo Fernández-Megía
Our research group is focused on the interface between organic and polymer chemistry with emphasis on the preparation of well-defined polymeric nanostructures for biomedical applications and the development of NMR tools for their characterization.
With this aim we rely on natural and synthetic polymers as well as on dendrimers. The efficient conjugation of these multivalent structures to ligands of biomedical interest opens the door to novel drug delivery and diagnosis agents. The characterization of the resulting nanostructures by NMR relaxation gives information on their structure and dynamics.
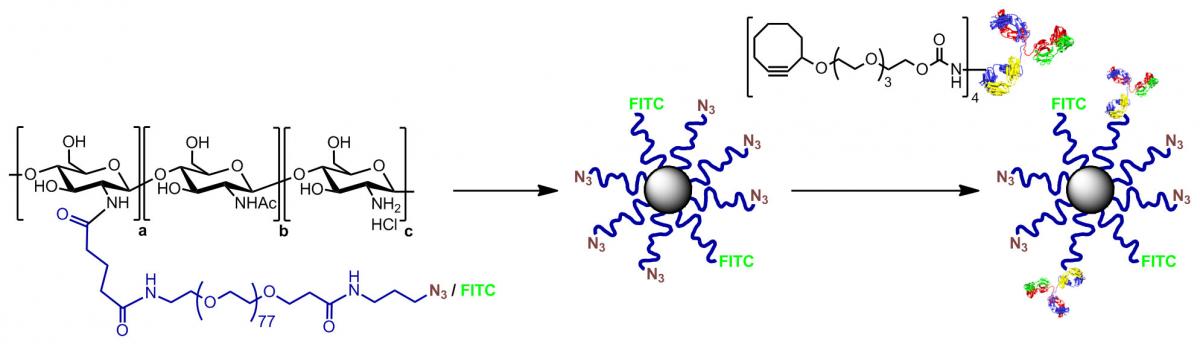
DENDRIMERS
Dendrimers are synthetic tree-like macromolecules of globular nature composed of repetitive layers of branching units. The multivalent presentation of chemical handles on their surface allows for their easy functionalization with carbohydrates and other biologically relevant ligands.
The resulting functionalized nanostructures have revealed as tools for the study of the multivalent carbohydrate receptor interaction (Surface Plasmon Resonance), the dynamics of dendrimers (NMR relaxation), and the preparation of polyion complex micelles and dendriplexes for drug and gene delivery applications.
Dendrimers have been also developed that interfere the aggregation of relevant HIV proteins and amyloid peptides, and as macromolecular contrast agents for Magnetic Resonance Imaging (MRI).
CHITOSAN AND DRUG DELIVERY
Chitosan (CS) is a natural polysaccharide characterized by a low toxicity and high biodegradability, that has plenty applications in the biomedical field. In order to understand the aggregation and dynamics of CS as a function of the degrees of acetylation and polymerization, fluorescence and rheology experiments have been performed that rule out the participation of the acetyl groups in the process.
With the aim of increasing the poor solubility of CS at physiological pH, our group has prepared PEG-grafted CS copolymers (CS-g-PEG) which combine the unique properties of CS and the solubility of PEG.
The decoration of CS-g-PEG with ligands by means of efficient coupling technologies allows the preparation of functionalized nanostructures for advanced applications in drug delivery as demonstrated in collaboration with various research groups worldwide.
NMR RELAXATION
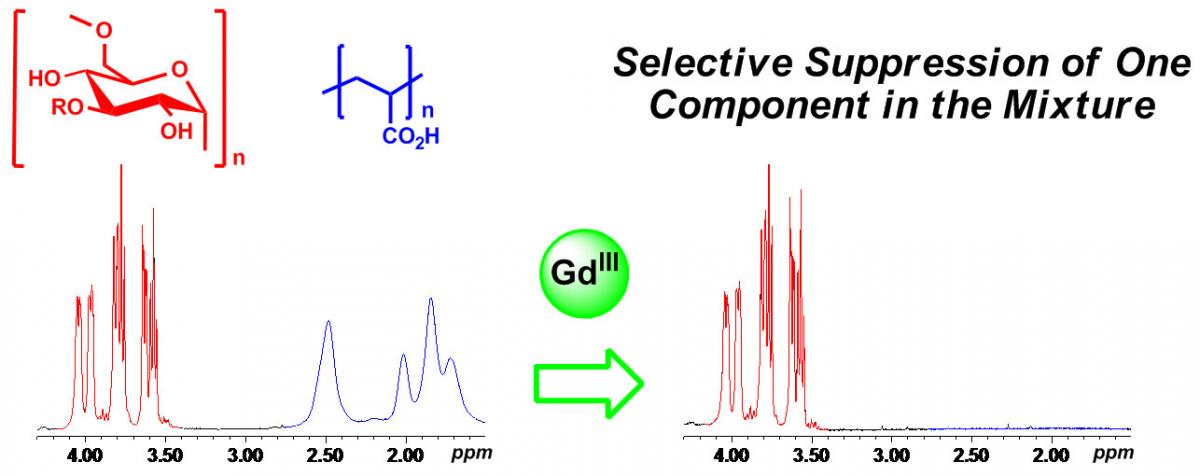
The development of NMR tools for the characterization of polymers and polymeric nanostructures is another area of interest in our group. We have recently described the use of paramagnetic relaxation agents for the reduction of NMR acquisition times and the selective suppression of signals in the spectra of complex polymeric mixtures.
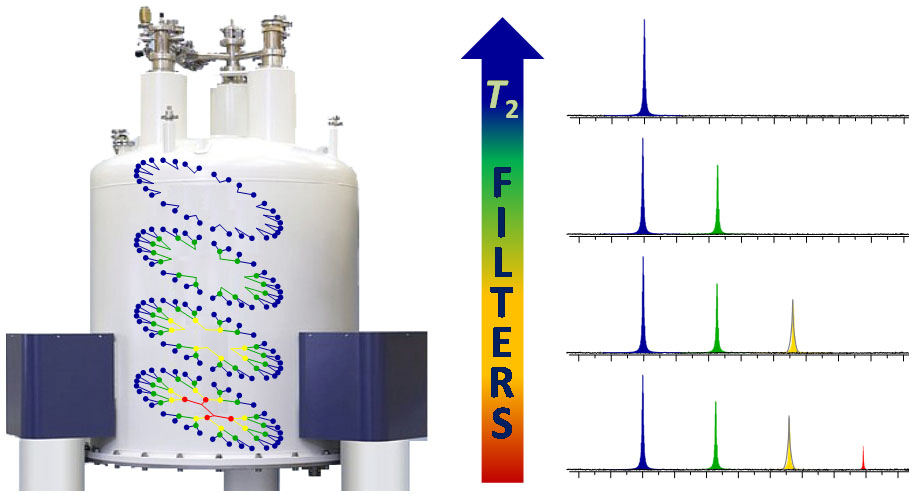
In addition, relaxation times can be exploited to gain relevant information about the relative dynamics of polymers and dendrimers. In the particular case of dendrimers, the characteristic distribution of transverse relaxation times (T2 shorter at the core than the periphery) can be exploited in T2-edited 1D and 2D NMR experiments for the stepwise filtering of internal nuclei according to their topology within the dendritic structure.
STIMULI-RESPONSIVE DYNAMIC POLYMERS: Helical Polymers, Sensors and Nanostructures
Supervised by Prof. Ricardo Riguera, Prof. Emilio Quiñoá and Prof. Félix Freire
(Ver vídeo "Nuevos Materiales Basados en Polímeros Helicoidales")

The possibility of controlling and changing the helicity and elongation of polymers through external stimuli (temperature, solvent, light, etc.) allows their use as sensors, molecular devices, chiral catalysts and memory elements, among other applications.
In order to manipulate the structure of these polymers (e.g., elongation and helical sense), it is necessary to identify the crucial structural parameters that must be modified to achieve the desired objectives through an appropriate design. The knowledge of these parameters requires a great mastery of the different techniques that allow obtaining structural data on the helical polymers.
In addition, it is necessary to study the different transmission mechanisms of the responses created by the stimuli on the set of monomeric repetition units, which may be of the "amplification of chirality", "helicity reversal", "sergeants and soldiers effect", "chiral conflict", "chiral coalition" type, etc., depending on whether one works with homopolymers or copolymers.
In our research group we are interested in the nanostructuration of these macromolecules and in the formation of hybrid materials through their association with metal nanoparticles, DNA or proteins.
Some of our most interesting contributions in this field are:
STRUCTURAL ANALYSIS OF HELICAL POLYMERS
In our research group we are very interested in the structural analysis of helical polymers, trying not only to use the classic characterization techniques (CD, AFM), but also seeking to introduce new protocols (preparation of monolayers, VCD, ROA, DSC, DFT calculations or ab initio) that allow obtaining more structural information of these complex systems.
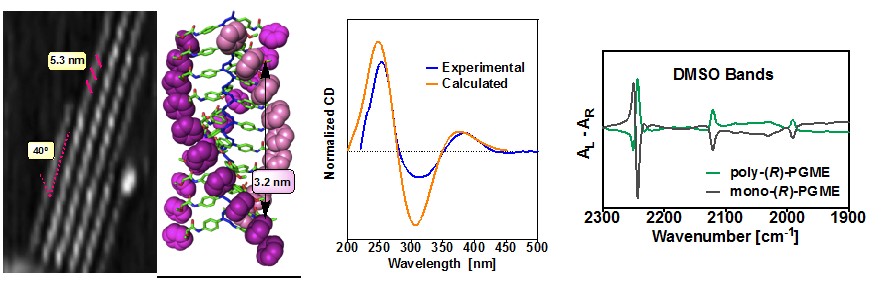
Angew. Chem. Int. Ed., 2018, 57, 3666 J. Am. Chem. Soc., 2016, 138, 9620
SELECTION OF THE HELICAL SENSE AND ELONGATION IN THE POLYMER
We have described the reversible control of helicity and elongation of polyphenylacetylenes through dynamic coordination chemistry and cation-π type interactions. The different and controlled coordination of metal cations to the monomeric repeat units in combination with the activation/deactivation of supramolecular interactions, such as cation-π, allows generating multiple helical structures from a single starting material.
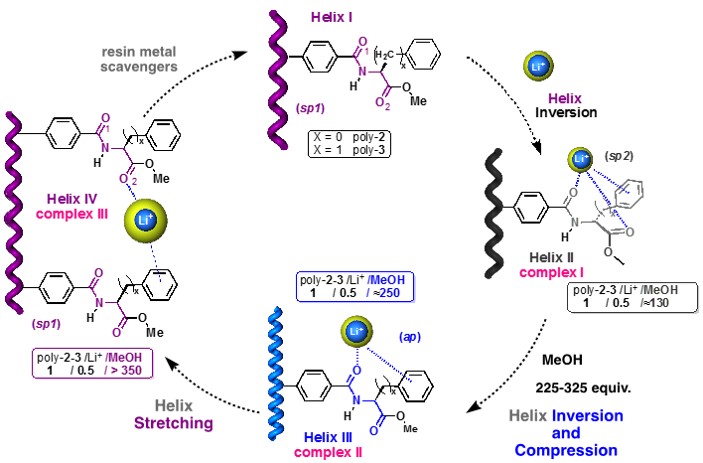
Currently, we continue working in this field using different stimuli (e.g., light, temperature, pH, chiral molecules) to cause changes in the structure of the polymers and thus be able to be used as sensors.
NANOSTRUCTURATION AND HYBRID MATERIALS
The use of metal ions to control the helical sense and the elongation of helical polymers has allowed us to generate chiral nanostructures of controlled size and chirality, where the metal ion acts as a crosslinking agent.
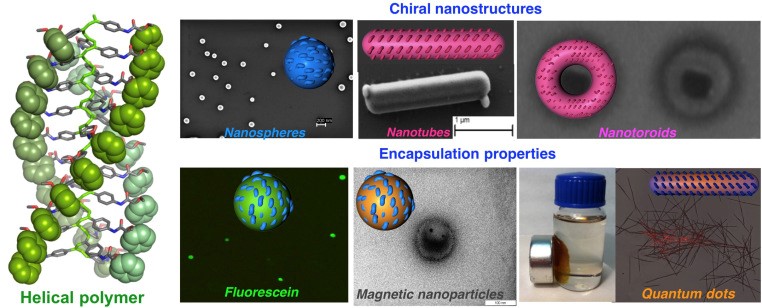
Angew. Chem. Int. Ed., 2014, 53, 13720
In addition, we are studying the self-assembly of helical polymers to give rise to well-defined three-dimensional structures: fibers, polymeric vesicles, gels, etc.
The introduction of monomer repeating units with functional groups with supramolecular recognition capacity will allow us to study the formation of new materials based on the interaction between polymer chains (stereocomplexes) or even the formation of hybrid materials by association with biopolymers —for example, (peptide/DNA)/helical polymer—.
Another line of research that we are carrying out is the formation of hybrid materials formed by metallic gold or silver nanoparticles and helical polymers in order to study the properties of these systems and the formation of a chiral plasmon.
COMMUNICATION MECHANISMS BETWEEN CO-MONOMERS OF A COPOLYMER
The amplification of a helical sense by copolymerization with a chiral monomer of an axially racemic polymer, formed by an achiral monomer, is widely known, this phenomenon being called "Sergeants and Soldiers effect". In our research group we studied the activation of the chiral co-monomer resorting to the use of external stimuli.
In addition, we are interested in knowing if these mechanisms of communication between monomers are also produced in chiral copolymers, analysing how the different absolute configurations of the Sergeant and the Soldier affect and modulate the different chirality amplification mechanisms.
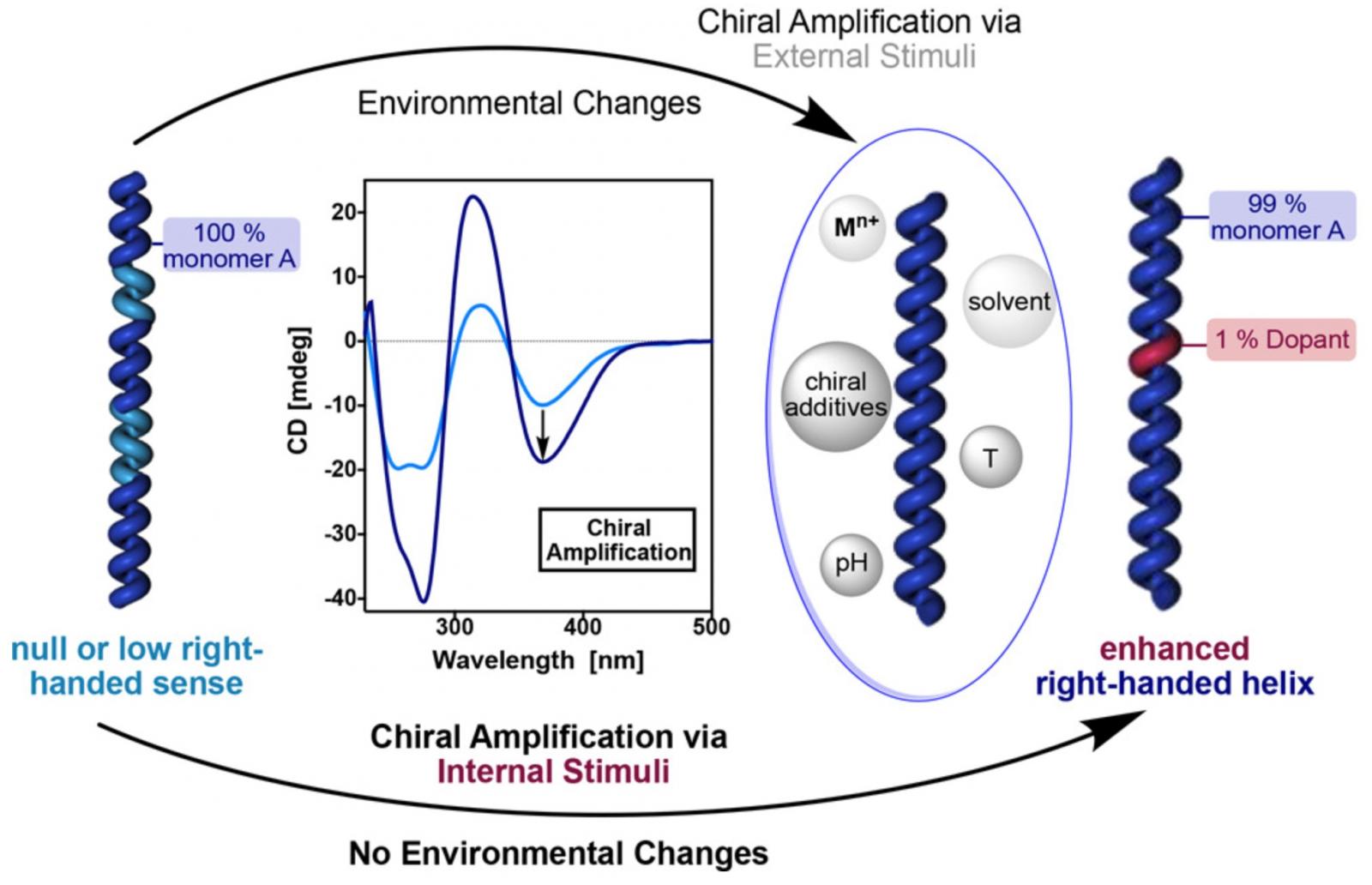
J. Am. Chem. Soc., 2018, 140, 667
CARBOHYDRATE CHEMISTRY
Supervised by Prof. Juan Carlos Estévez Cabanas and by Prof. Ramón José Estévez Cabanas
Carbohydrate Chemistry is a powerful synthetic tool that allows access to a wide variety of polyfunctionalised molecules, mainly polyhydroxylated, which are difficult to reach by other synthetic routes. These molecules, mostly carbocycles and heterocycles, present several functional groups arranged in a perfectly defined spatial distribution, which can not only be transformed into other groups, but can also be easily modulated in their polarity by simply protecting or deprotecting hydroxyl groups. Due to this versatility, these molecules are a chemical tool with great potential in areas such as Biological Chemistry or Medicinal Chemistry; as well as in the area of Molecular Materials.
Our research group explores the generation of small polyhydroxylated molecules with biological activity including: imono sugars, polyols and polyhydroxylated amides.The synthesis of polyfunctionalised amino acids, the study of their incorporation into peptides (homooligomers and heterooligomers) and the secondary structure of these peptides in solution; totally novel compounds in terms of their structural properties and their application us chemists and as new biological and/or industrial materials.
We are also developing a line of research on gelling agents (lipogels and hydrogels) based on the synthesis of amides and peptides from non-natural amino acids, polyfunctionalised or not, of interest in the administration of drugs, the recovery of contaminated water, etc.
Given the strong relationship that our research has with the discovery of new molecules capable of modifying the behaviour of water, we have begun to explore a new research topic based on the synthesis of molecules capable of inhibiting the recrystallisation of ice (IRI), a topic that, in our opinion, is under-researched in the Spanish research system, despite its enormous importance for the advancement of medicine and its important industrial and environmental applications.
Small polyhydroxylated molecules with biological activity
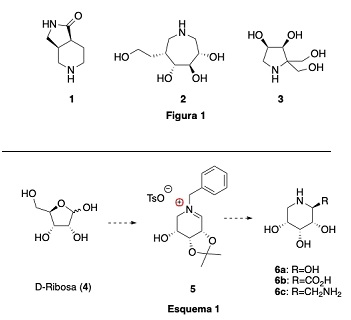
Starting from carbohydrates and other commercial polyhydroxylated products, such as (-)-Shikimic acid, biologically active compounds can be generated, mainly modified imino-sugars, but also polyhydroxylated amino alcohols, polyols and polyfunctionalised amides, capable of improving the properties of existing ones and/or offering new therapeutic targets for the treatment of metabolic diseases, a research topic in which our group has made contributions of interest to medicinal chemistry, such as the synthesis of:
• Azabicyclic amide 1, an HIV integrase inhibitor, obtained from (-)-shikimic acid.
• Imino-sugar 2 (a glycosidase inhibitor) obtained from D-glucose.
• The new generation imino-sugar 3, obtained from D-glyceraldehyde.
T. Asym. 2015, 26, 320-23 Org. Lett. 2007, 9(4), 623-26
Currently, we continue to work on this line of research, exploring a new strategy based on the transformation of D-ribose (4) into polyhydroxylated amino acids 6b and second-generation imino-sugars 6c, via iminium salts 5 (Scheme 1).
Polysubstituted cycloalkane amino acids and their oligomers
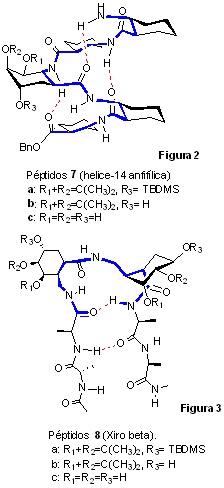
Our research group is also working on the synthesis of new polysubstituted amionacids, preferably of β- and γ-cycloalkane nature, as well as on their incorporation into their homo- and heterooligomers and the study of their properties, with special emphasis on their folding properties in solution (foldamers). In this field we have found results that, although preliminary, are of extraordinary interest.
• Thus, we have synthesised the pentapeptides 7a-c, constituted by unsubstituted β-cyclohexane β-amino acids with a polysubstituted β-cyclohexane β-amino acid (synthesised in our research group from (-)-shikimic acid), demonstrating that they are structured in solution giving rise to a 14-helix, this being the first time that a helical structure has been achieved in water with a polyhydroxylated oligomer (7c. Figure 2). This result, although preliminary, is important because depending on the proportion of each type of amino acid (unsubstituted and polyhydroxylated) new amphiphilic helical structures of great interest as new materials can be generated.
• We have also synthesised oligomers 8a-c (Figure 3) constituted by L-alanine units and two polysubstituted cyclohexane γ-amino acids (synthesised in our research group from (-)-Shikimic acid), demonstrating that these peptides show a strong tendency to fold as beta turns, another of our preliminary results of great interest for the generation of artificial proteins.
We are now working on the implementation of these results in the generation of more complex peptides than the previous ones, which maintain their structural properties and can be applied to the generation of new materials.
JOC 2018, 83, 1543-50 ACS Omega 2022, 7, 2002-14
Gelling agents, (lipogels and hydrogels)
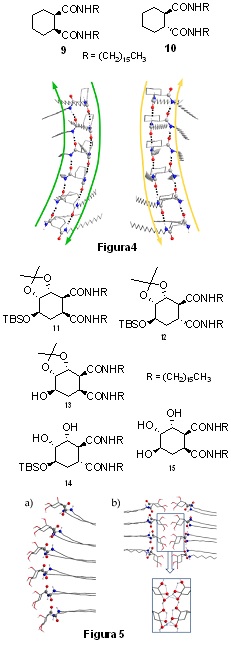
We are also developing a research line on gelling agents (lipogels and hydrogels) based on the synthesis of amides and peptides of a cycloalkane nature, with or without substitution in their cyclic system, of interest in the administration of drugs, the recovery of contaminated water, etc. In this field:
• We have synthesised bisamides 9 and 10 and studied their behaviour as gelling agents in different solvents. Both bisamides have shown moderate to good gelling ability in apolar solvents, being especially relevant that both compounds generate chiral aggregates (Figure 4) despite the fact that one of them is a meso molecule, a new example of stochastic symmetry breaking induced by sonication.
• We also developed applications based on bisamides 11-15 derived from trihydroxycyclohexane-1,2-dicarboxylic acids (synthesised in our research group from (-)-shikimic acid); whose hydroxyl groups can be free or, alternatively, present full or partial protection.
These amides showed a relationship between their gelation ability and the substitution pattern of their hydroxyl groups. While compounds with fully protected hydroxyl groups (11 and 12) are good organogelators for alcohols (methanol, ethanol, isopropanol), organogelators with one or two free hydroxyl groups (13 and 14) show an ambivalent ability to gel both apolar and polar solvents. The compound with three free hydroxyl groups (15) is insoluble in water and polar solvents, including alcohols, but is able to gel in low polar solvents.
This seemingly random behaviour could be rationalised by assuming that compounds with fully protected hydroxyl groups adopt a gerarchical order based on the strong hydrogen bonding between the amide groups of the organogelator molecules (Model a. Figure 5). Whereas compounds with partially or fully unprotected hydroxyl groups can adopt the above structure or a completely different one based on the strong hydrogen bonding between the free hydroxyl groups of the organogelator molecules (Model b. Figure 5), depending on the polarity of the solvent.
Eurojoc 2017, 23, 3357–3365 Molecules 2019, 24, 352
We continue to work in this field of new lipogels and hydrogels by exploring the behaviour of different amides with cycloalkane structure.
Ice Recrystallisation Inhibition (IRI)
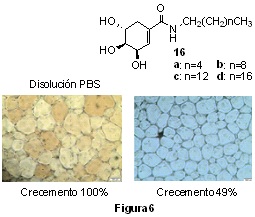
More preliminary results, but extremely interesting, are being obtained in the new line of research initiated by our group based, as in the case of hydrogels, on the modification of the properties of water.
This involves the generation of new molecules capable of inhibiting the recrystallisation of ice (IRI agents), a field of research of enormous interest for medicine, but also for food and other areas such as the environment.
This project began with the alkylamides (16a-d), synthesised in our research group from (-)-Shikimic acid), and the study of their IRI properties. This allowed us to demonstrate that octylamide 16b (Fig 6) inhibits ice recrystallisation by 49%; an important result for the work we are now developing with the implementation of derivatives of this molecule with better IRI properties.