-
A new CiQUS collaboration with IBM and an international team of researchers now featured on the cover of the journal Science. Scientists have found a method to transform the structure of individual molecules, controlling the forming and cleaving of bonds by tip-induced chemistry.

The work has been featured on the cover of Science | AAAS Science.
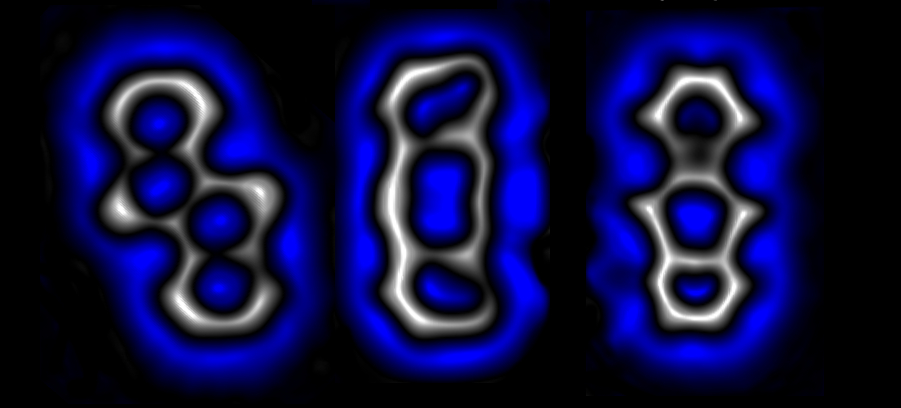
Selectively and reversibly the structure in the center can be transformed to the structure on the right or on the left. Images of the molecules obtained by high-resolution atomic force microscopy | IBM Research
A new collaboration between Center for Research in Biological Chemistry and Molecular Materials (CiQUS), IBM researchers and an international team have demonstrated the selective and reversible formation of intramolecular covalent bonds by tip-induced chemistry. Such control is unprecedent and opens a route towards advanced artificial molecular machines. The work is now featured on the cover of the journal Science.
In molecules, atoms are linked together to form a three-dimensional, nanometric structure. Molecules with the same number and type of atoms may present their bonds in different ways, with different connectivity between their atoms. These compounds, known as structural isomers, bring extraordinary variability to the molecular world. Now, researchers have found a method to transform one structural isomer into another by reconnecting their bonds at will in response to an external stimulus. Using a voltage pulse from the tip of a scanning tunneling microscope (STM) , scientists induced very accurate transformations in the structure of a molecule formed by four carbon rings.
"Since the 19th century, chemists have been trying to manipulate the connectivity between atoms within a molecule in order to obtain new functionalities" said Diego Peña, Principal Investigator at CiQUS and coauthor of the study. "We can now do this extremely precisely and on individual molecules, as if we were using nanometric clamps". The work is a collaboration between CiQUS and researchers from IBM Research, King Abdullah University of Science and Technology (KAUST) and Universität Regensburg. "The reactions are reversible, therefore we can switch between all these molecular structures repeatedly and controlled" said Leo Gross, IBM researcher and coauthor of the study: "Such selective and reversible bond formation could enable molecular machines that perform variable and complex tasks".
Molecular machines are molecules that perform a task in response to an external stimulus. For example, the human body houses a large number of molecular machines with critical functionality for life, as in DNA replication. The design and synthesis of artificial molecular machines in laboratory is a very complex task, which earned Jean Pierre Sauvage, J. Fraser Stoddart and Ben L. Feringa the 2016 Nobel Prize. The making and breaking of bonds inside a given molecule is not only of very fundamental interest to chemistry, but it also entails a control of the molecule's structure and this, in turn, is the basis for molecular machines: controlling the structure of a given molecule at will. Peña explains that "until now, artificial molecular machines have mainly relied on transformations in the spatial distribution of atoms induced by external stimuli. By adding control over the connectivity between atoms, we can address more complex designs". His team is involved in an European Research Council project focusing on the manipulation of single molecules (MolDAM - ERC SyG). In the next steps, researchers contemplate triggering the reactions by light or transferring electrons between different sites within the molecule, rather than by the tip of an STM microscope.
Reference
F. Albrecht, S. Fatayer, I. Pozo, I. Tavernelli, J. Reep, D. Peña, L. Gross. Selectivity in single-molecule reactions by tip-induced redox chemistry. Science, 2022, 377, 298-301


