Mercedes Novo and Wajih Al-Soufi
Dye micelle interaction
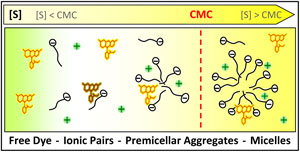
The interaction of the moderately hydrophobic cationic dye R123 with surfactants of differently charged head groups shows a behaviour of surprising variety. The simplest case is found with nonionic surfactants. Below the CMC, dye and surfactant molecules do not interact, whereas, once micelles are formed, the dye exchanges between the aqueous and the micellar phases with a partition equilibrium constant which depends on the strength of the hydrophobic interaction with the specific surfactant. In the case of cationic surfactants the behaviour is, at first sight, similar. The partition equilibrium constant is much lower than in the case of nonionic micelles, due to the electrostatic repulsion between dye and head groups. However, now the counterions play an important role acting as effective fluorescence quenchers already below the CMC. The most complex situation presents the interaction with anionic surfactants. The strong electrostatic attraction between dye and surfactant leads to the formation of ionic pairs with low quantum yield and reduced solubility at very low surfactant concentrations. At higher concentrations more and more small dye-surfactant aggregates are formed with photophysical properties similar to those of the dye bound to fully formed micelles. Above the CMC all the dye is included in micelles.
Role of electrostatic and hydrophobic forces in the interaction of ionic dyes with charged micelles
Sonia Freire, Jorge Bordello, Daniel Granadero, Wajih Al Soufi and Mercedes Novo, Photochemical & Photobiological Sciences 9(5): 687-696.
Dye exchange dynamics
 Micelles can also be considered as soft supercages in which host molecules can enter yielding supramolecular complexes, for which the association/dissociation dynamics are of critical importance. Like membranes, micelles are highly cooperative, dynamic, organized molecular assemblies, where hydrophobic interactions play an important role. As model systems they are simpler, with well-defined sizes and advantageous properties.
Micelles can also be considered as soft supercages in which host molecules can enter yielding supramolecular complexes, for which the association/dissociation dynamics are of critical importance. Like membranes, micelles are highly cooperative, dynamic, organized molecular assemblies, where hydrophobic interactions play an important role. As model systems they are simpler, with well-defined sizes and advantageous properties.
We study the exchange dynamics by means of fluorescence correlation spectroscopy (FCS), using micelles as membrane mimetic systems and fluorescence markers, such as rhodamine 123 (R123) or coumarine 153 (C152), as probes. We confirm that micelles act as soft-cages with a diffusion controlled rate constant of the dye-micelle association.
Concentration-model for surfactants near the cmc
![Normalized physical–chemical properties of an aqueous SDS solution as function of total surfactant concentration [S]0 with the parameters determined from the fits to the experimental data presented above using the concentration model.](/fotofqm/sites/default/files/investigacion/fig-7.png)
We present a model for the concentrations of monomeric and micellized surfactants in solution as a consistent approach for the quantitative analysis of data obtained with different experimental techniques from surfactant solutions.
The concentration model provides an objective definition of the critical micelle concentration (cmc) and yields precise and well defined values of derived physical parameters. The use of a general concentration model eliminates subjective graphical procedures, reduces methodological differences, and thus allows one to compare directly the results of different techniques or to perform global fits.
We use the concentration model for the analysis of properties such as electrical conductivity, surface tension, NMR chemical shifts, absorption, self-diffusion coefficients, fluorescence intensity and mean translational diffusion coefficient of fluorescent dyes in surfactant solutions.
The fluorescence emission of pyrene in micellar solutions (fluorescence intensity, band ratio, excimer formation and fluorescence lifetimes) are studied in detail.
Examples of the curve fitting with Origin, Excel and Python
References
A Model for Monomer and Micellar Concentrations in Surfactant Solutions. Application to Conductivity, NMR, Diffusion and Surface Tension data
Wajih Al-Soufi, Lucas Piñeiro, Mercedes Novo, Journal of Colloid and Interface Science 2012, 370, 102–110 DOI: 10.1016/j.jcis.2011.12.037
Dye exchange in micellar solutions. Quantitative analysis of bulk and single molecule fluorescence titrations
Lucas Piñeiro, Sonia Freire, Jorge Bordello, Mercedes Novo, and Wajih Al-Soufi, Soft Matter 2013, 9, 10779-10790, DOI: 10.1039/ c3sm52092g
Fluorescence Emission of Pyrene in Surfactant Solutions
Lucas Piñeiro, Mercedes Novo, and Wajih Al-Soufi, Advances in Colloid and Interface Science 2015, 215, 1–12. DOI:10.1016/j.cis.2014.10.010
A Surfactant Concentration Model for the Systematic Determination of the Critical Micellar Concentration and the Transition Width (Review)
Wajih Al-Soufi and Mercedes Novo, Molecules 2021, 26, no. 17: 5339. https://doi.org/10.3390/molecules26175339



