Host-Guest complexes are supramolecular aggregates stabilized by non-covalent bonds and are ubiquitous in biochemical, pharmaceutical, environmental, cosmetic, food, and other chemical systems and applications. Different approaches are necessary in order to fully understand a supramolecular system: structural studies yield stoichiometry, geometry, association sites and heterogeneity, thermodynamic studies provide information about stability, equilibrium constants and reversibility, and finally dynamic studies are necessary to describe the association dynamics, diffusional properties or conformational dynamics. Most published studies focus on structure and thermodynamics of supramolecular association, which, however, give very little information about the dynamics of the system.
It is our aim to exploit Fluorescence Correlation Spectroscopy (FCS) for the study of supramolecular association and, more specifically, of the stability and the binding dynamics in host-guest systems. We show how knowledge about the dynamics gives valuable information about the association mechanism, which is directly related to the structure of the host and the guest.
Stability of Cyclodextrin Inclusion
Cyclodextrins are naturally occurring water-soluble toroidally shaped polysaccharides with a highly hydrophobic central cavity that have the ability to form inclusion complexes with a variety of organic and inorganic substrates. The three major cyclodextrins are the alpha, beta and gamma CD’s built up from 6, 7 and 8 glucopyranose units, respectively. CDs are often found as building blocks of supramolecular systems and in self-assemblies. The inclusion complexes result from specific noncovalent interactions between CDs and guest molecules, being therefore also simplified models for the study of molecular recognition phenomena of great importance in host-ligand binding in biological systems. Moreover, the ability of CDs to form inclusion complexes, in which the physicochemical properties of the guest molecules change with respect to the free molecules, has led to their widespread industrial, use and to applications in green chemistry.
Specific interactions in the inclusion complexes of pyronines Y and B with gamma and beta-cyclodextrin.
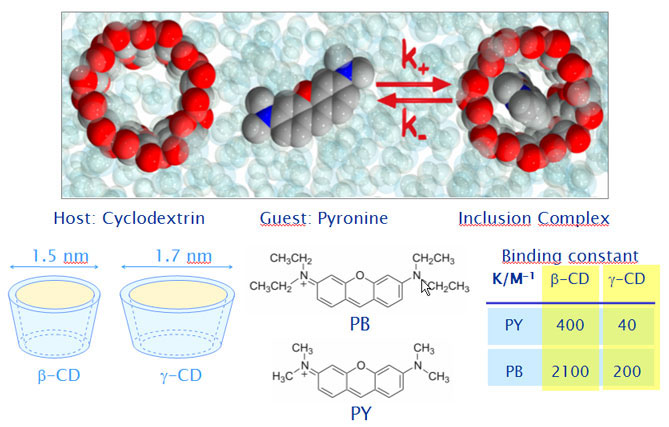
As guest molecules the dyes Pyronine Y (PY) and Pyronine B (PB) are studied (Figure 1). They provide simplified models of the xanthene skeleton of rhodamines, and are also used in the labeling of proteins and cell organelles. In photophysical studies on the complexation of PY and PB with beta-CD (bCD) and gamma-CD (gCD) we found that at low pyronine concentration both pyronines form 1:1 complexes with bCD and gCD. Complexation causes a slight red shift of the emission spectra of the pyronines, but decreases significantly their fluorescence quantum yields and lifetimes. The association equilibrium constant (binding constant) of PB to the CD’s is much higher than that of PY (see table in Figure1). This is a surprising result since it is presumed that the more bulky PB would show stronger steric hindrance than PY to enter into the CD cavity. Comparison of the photophysical properties of the pyronines in different solvents with those of the complexes suggested that there are important specific interactions of the pyronines with the electron-rich oxygens present in the interior of the CD cavity stabilizing the complexes formed with PB more than PY. With both pyronines this stabilization is much weaker in the case of gCD as compared to bCD. In order to confirm this hypothesis we compared the complexation dynamics of these pyronines with both CDs and analyze the individual steps during association and dissociation (see below).
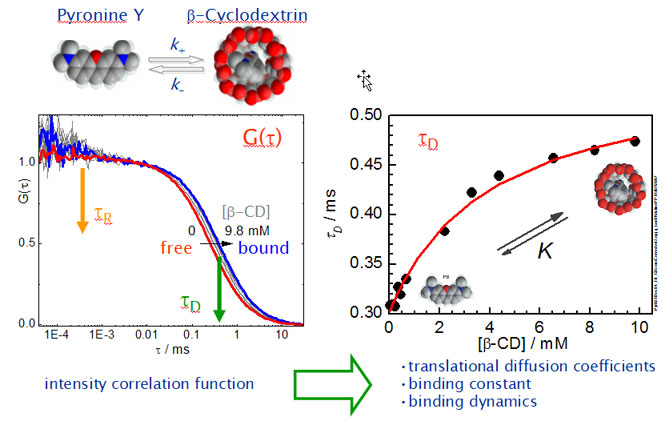
Dynamics of Cyclodextrin Inclusion
Whereas the thermodynamic and structural properties of cyclodextrin host-guest complexes have been extensively studied, the complexation dynamics have been addressed in much less extent. The dynamics of complex association and dissociation, however, is not only of fundamental interest for a better mechanistic understanding of the complexation process itself - a task by far not reached yet - but are also the key to comprehend and to control the huge variety of functions that CDs can fulfill.
We study the complexation dynamics between cyclodextrins (CD) as hosts and two different pyronines as guests (see above).
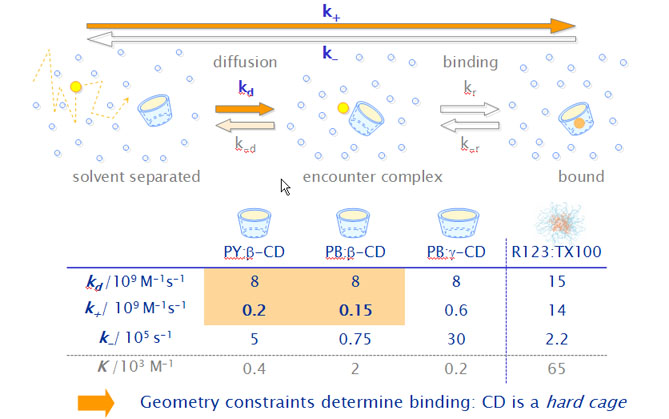
The estimated diffusion-limited rate constants kd are much higher than the observed association rate constants k+, which are limited by other processes than the diffusion itself. We assume therefore the formation of an encounter complex P·CD where P and CD are in the same solvent cage.
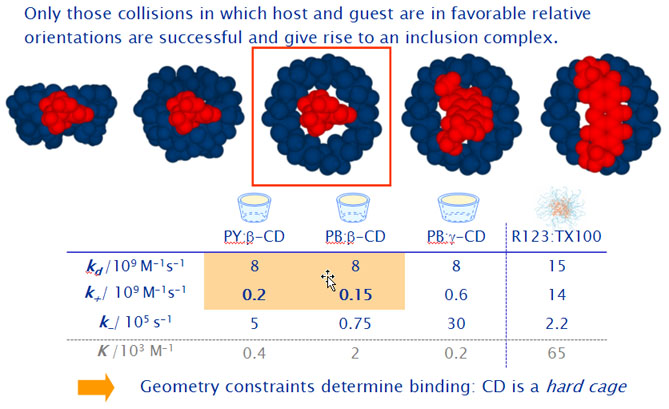
The estimated rate constants for the unimolecular inclusion process are equal for PY and PB.
- The unimolecular inclusion step limits the total de association rate.
- The different end groups have nearly no influence on this process.
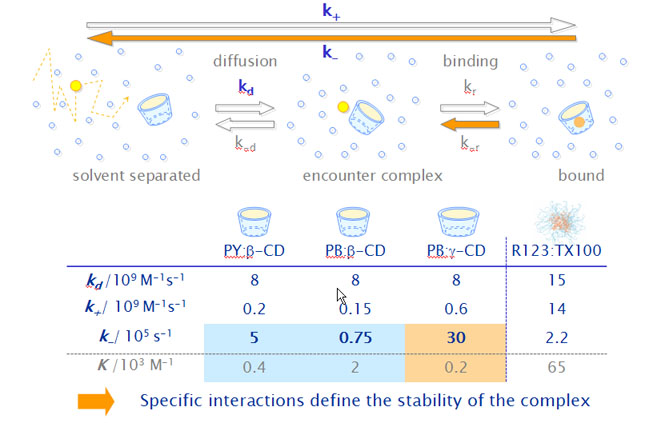
- The overall dissociation rate constant is limited by the rate constant k-r, with which the guest leaves the host forming again an encounter complex. This is the slowest of all involved steps.
- Specific interactions between the positively charged xanthene moiety of the pyronines and the electron-rich glucosidic oxygens of the b-CD cavity probably stabilize the inclusion complexes of both pyronines, but seem to be much stronger in the case of PB.
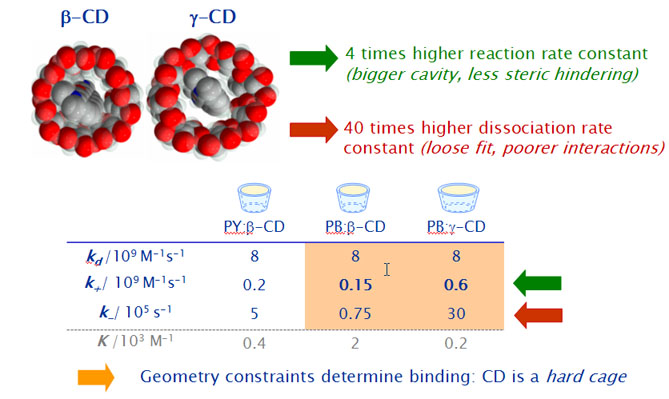
Results with the wider g-CD show in comparison to the smaller b-CD,
- a 4 times higher association rate constant in accordance to the higher inclusion probability predicted by the simulations.
- a 40 times higher dissociation rate constant due to poorer interactions between host and guest.
Both effects are responsible for the much lower stability equilibrium constants as compared to those with b-CD.



