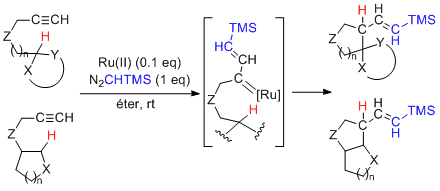
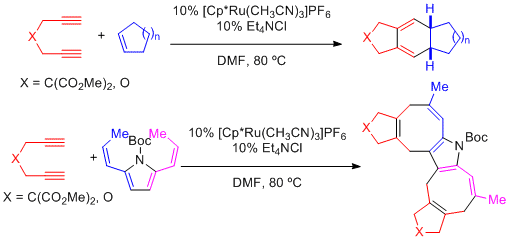
2.1 [2+2+2] and [4+2+2] cycloadditions of pi systems
Ruthenium-catalyzed reactions of alkenes (cyclic or acyclic) and 1,3-dienes to 1,6-diynes easily afford functionalized bicyclic 1,3-cyclohexadienes and 1,3,5-cyclooctatrienes via [2+2+2] and [4+2+2] cycloadditions.
Org. Lett. 2003, 5, 2841-2844
J. Am. Chem. Soc. 2006, 128, 9262-9263
Org. Lett. 2009, 11, 983-986
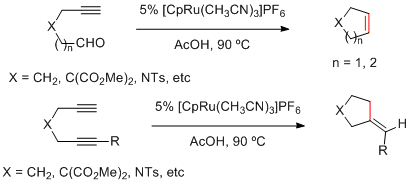
2.2 Carbo- and heterocyclizations
Functionalized cyclopentenes and cyclohexenes can be easily prepared by new Ru-catalyzed cyclization reactions of terminal alkynals and 1,6-diynes.
J. Am. Chem. Soc. 2006, 128, 9576-9577
J. Am. Chem. Soc. 2007, 129, 12916-12917
Heterocyclic units present in many bioactive compounds such as benzofurans, isochromenes, benzoxepines and dihydroisoquinolines can be easily synthesized by ruthenium- and osmium-catalyzed cycloisomerizations of aromatic alkynols and alkynamides. The key step is the nucleophilic attack of the heteroatom to the catalytic metal-vinylidene species generated in situ.
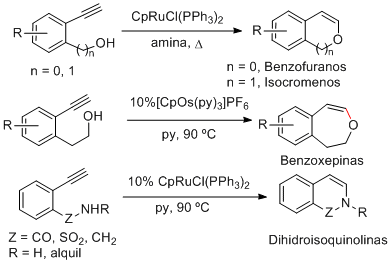
Org. Lett. 2009, 11, 5350-5353
Angew. Chem. Int. Ed. 2010, 49, 4278-4281
Adv. Synth. Catal. 2011, 353, 1933-1937
2.3 Brønsted acid-catalyzed carbocyclizations
Linear enyne acetals were efficiently converted into hydroazulene skeletons (found in many bioactive terpene derivatives) by a new Brønsted acid-catalyzed tandem reaction of carbocyclization/Nazarov cyclization. The process takes place in a stereospecific manner.

Org. Lett. 2009, 11, 1531-1533
Angew. Chem. Int. Ed. 2012, 51, 12316-12320