An article by CiQUS researchers Pablo del Pino and Beatriz Pelaz published in ACS Nano describe current challenges and recent advances in efficient delivery and targeting of nanoparticles in vivo.
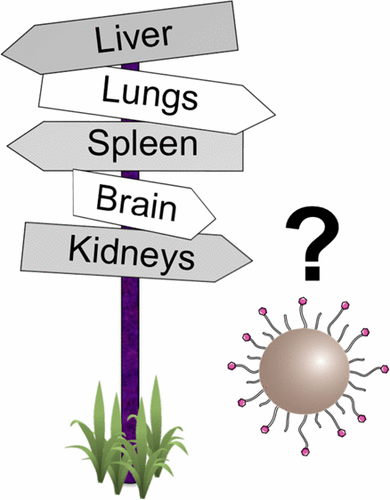
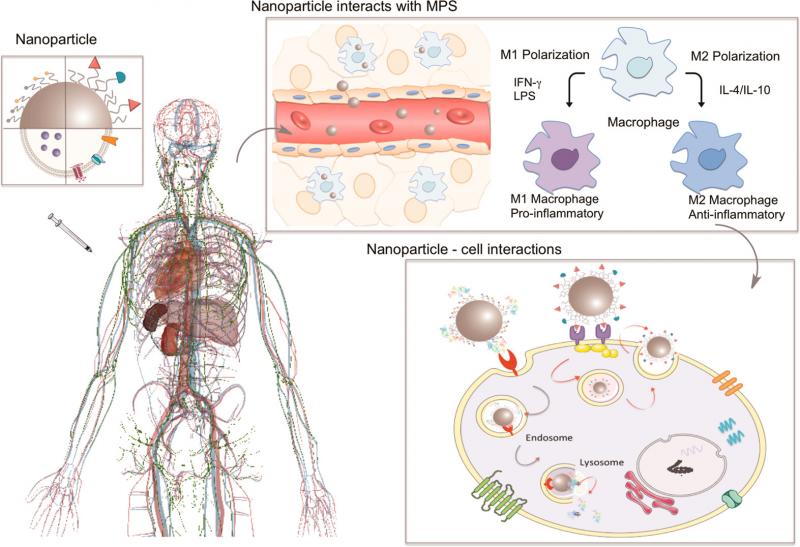 Since the discovery of the potential use of nanomaterials in the field of nanomedicine, scientists have tried to develop nano-drugs able to travel through the body and specifically recognize with great efficiency, for example, tumors. Unfortunately, more than 99% of nanoparticles administered in vivo are sequestered by the mononuclear phagocytic system (primarily by the liver and spleen) before reaching the tumors.
Since the discovery of the potential use of nanomaterials in the field of nanomedicine, scientists have tried to develop nano-drugs able to travel through the body and specifically recognize with great efficiency, for example, tumors. Unfortunately, more than 99% of nanoparticles administered in vivo are sequestered by the mononuclear phagocytic system (primarily by the liver and spleen) before reaching the tumors.
In this context, CiQUS researchers Pablo del Pino and Beatriz Pelaz, in collaboration with scientists from the University College of Dublin and the University Hospital of Santiago de Compostela, publish in the journal ACS Nano a perspective article where they discuss the latest advances and current challenges in order to enhance the capabilities of these nanoparticle-based drugs.
 It is now known that the rich complexity of the tumor microenvironment is now recognized not only as an integral factor contributing to carcinogenesis but also as playing critical roles modulating cancer therapy. Thus, this work highlights the real need for the development of nanomaterials libraries with well-defined and characterized physicochemical properties, to evaluate their behavior in cells, one in turn characterized in detail. With the development of basic studies of this nature, one can actually understand the interactions between cells and nanoparticles in vivo and, in turn, improve the design of nanoparticle-based drugs that are truly efficient.
It is now known that the rich complexity of the tumor microenvironment is now recognized not only as an integral factor contributing to carcinogenesis but also as playing critical roles modulating cancer therapy. Thus, this work highlights the real need for the development of nanomaterials libraries with well-defined and characterized physicochemical properties, to evaluate their behavior in cells, one in turn characterized in detail. With the development of basic studies of this nature, one can actually understand the interactions between cells and nanoparticles in vivo and, in turn, improve the design of nanoparticle-based drugs that are truly efficient.
The authors also emphasize that the barrier between proof of concept and advanced medicines based on nanomaterials (beyond clinical trials) will narrow as far as grows our knowledge about the interactions between nanomaterials and biomolecules within living beings, much more complex than in cell cultures.
Note
In the same issue, MacParland et al. shed some light on hepatic sequestration of nanoparticles, the major nonspecific target organ (together with the spleen) of administered nanoparticles.


