-
CiQUS researchers have developed a broad-spectrum inhibitor to combat infections caused by P. aeruginosa, the paradigm of bacterial resistance to antibiotics.
-
The compounds developed by Prof. González Bello's group are able to restore the activity of ceftazidime, the most widely used antibiotic for the treatment of infections caused by this bacterium.
-
The study was recently published in the Journal of Medicinal Chemistry and conducted in collaboration with the Complejo Hospitalario Universitario A Coruña (CHUAC) and scientists from the Instituto de Investigación Sanitaria Illes Balears (IdiSBA).
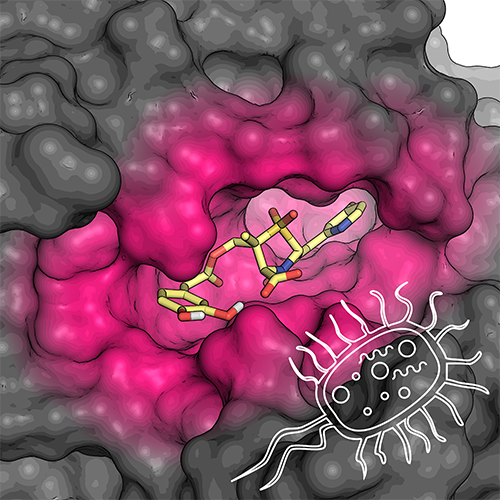
Image: Gonzalez-Bello group
References
J. C. Vázquez-Ucha, D. Rodríguez, C. Lasarte-Monterrubio, E. Lence, J. Arca-Suarez, M. Maneiro, E. Gato, A. Perez, M. Martínez-Guitián, C. Juan, A. Oliver, G. Bou, C. González-Bello, and A. Beceiro. 6-Halopyridylmethylidene penicillin-based sulfones efficiently inactivate the natural resistance of Pseudomonas aeruginosa to β-lactam antibiotics. J. Med. Chem. 2021, 64, 6310-6328 (doi: 10.1021/acs.jmedchem.1c00369)
D. Rodríguez, M. Maneiro, J. Vázquez-Ucha, A. Beceiro, and C. González-Bello. 6-Arylmethylidene Penicillin-based Sulfone Inhibitors for Repurposing Antibiotic Efficiency in Priority Pathogens. J. Med. Chem. 2020, 63, 3737-3755
Recent article published in Journal of Medicinal Chemistry shows penicillin-based sulfones ability to tackle infections caused by Pseudomonas aeruginosa, a paradigm of antimicrobial resistance and a major cause of hospital-acquired infections. These compounds, particularly a bromide developed at Center for Research in Biological Chemistry and Molecular Materials (CiQUS), can restore the activity of ceftazidime, the most widely used antibiotic for the treatment of P. aeruginosa infections. The study was conducted in collaboration with Complexo Hospitalario Universitario A Coruña (CHUAC) and Institut d´Investigació Sanitària Illes Balears (IdiSBA).
In recent years, the number of superbugs that have become drug-resistant has increased dramatically. COVID-19 seems to have magnified the problem, as patients become susceptible to a secondary bacterial infection that can only be treated with antibiotics. At the same time, the high use of antibiotics is increasing drug resistance, making the need for new therapies and strategies to tackle these pathogens urgent.
In this context, penicillin-based sulfones developed in Prof. González Bello´s group have demonstrated to restore the efficacy of the most relevant broad-spectrum antibiotics in clinical use. These compounds have a very unique mechanism of action “without destroying the bacteria, because they are not antibiotics but antibiotic enhancers, i.e. they help the drug to perform its function efficiently” said CiQUS researcher: “these compounds act by blocking the most widespread and common resistance mechanism in multidrug-resistant pathogenic bacteria, the antibiotic breakdown, the hydrolysis, through β-lactamase enzymes“
In a previous research highlighted in the Journal of Medicinal Chemistry cover, the authors found that such compounds restore the activity of carbapenems, being a bromopyridyl derivate the most promising candidate. Through antimicrobial susceptibility studies, proteomics and molecular dynamics simulations this new work confirmed that the bromide is also able to restore the efficacy of ceftazidime, the most widely used antibiotic for the the most widely used antibiotic for the treatment of P. aeruginosa. infections.
This bacterium possesses a chromosomal class C β-lactamase known as PDC which can be induced in most clinical isolates by some of the β-lactam antibiotics. This gives P. aeruginosa the ability to regulate the level of resistance on demand via production of the AmpC, thus making the bacterium intrinsically resistant to β-lactam antibiotics. Compounds developed at CiQUS, particularly the bromide, can block this process even when the bacteria significantly increase its production, thus allowing the antibiotic to operate normally. In addition, molecular dynamics simulation studies revealed that these compounds affect an enzyme pocket which is not fully evident in all cases. This pocket is responsible for its excellent efficacy, far superior to the inhibitors currently used in the clinical context. Penicillin-based sulfones developed in CiQUS therefore perform as broad-spectrum inhibitors, restoring the activity of cephalosporins and cabapenems in clinical use against multi-resistant pathogens by using a novel mechanism of action that allows the enzyme to be blocked for much longer and more effectively.
This antibiotic-resistance inhibitor therapy has great advantages. On the one hand, it allows reuse of the excellent arsenal of antibiotics that are safe, highly effective and well tolerated by most patients. On the other hand, it prevents us from having to devote a great deal of effort to the challenging process of identifying and validating new therapeutic targets” said Gonzalez-Bello.


