Her doctoral thesis is focused on the development of novel antibiotics for the treatment of bacterial infections whose mode of action is based on the selective and efficient inhibition of one of the enzymes involved in the biosynthesis of the aromatic amino acids, shikimate kinase.
 CiQUS´s PhD student Verónica Prado López has defended today her doctoral thesis entitled “Inhibitors of the Shikimate Kinase Enzyme for the Treatment of Bacterial Infections: Design, Synthesis and Biological Evaluation”, under the supervision of Concepción González Bello, associate professor and principal investigator at the center.
CiQUS´s PhD student Verónica Prado López has defended today her doctoral thesis entitled “Inhibitors of the Shikimate Kinase Enzyme for the Treatment of Bacterial Infections: Design, Synthesis and Biological Evaluation”, under the supervision of Concepción González Bello, associate professor and principal investigator at the center.
Her doctoral thesis is focused on the development of novel antibiotics for the treatment of bacterial infections, whose mode of action is based on the selective and efficient inhibition of one of the enzymes involved in the biosynthesis of the aromatic amino acids, shikimate kinase. This enzyme is essential in relevant pathogenic bacteria such as Mycobacterium tuberculosis, the bacillus that causes tuberculosis and Helicobacter pylori, which is responsible for chronic gastritis and is associated with gastroduodenal ulcers. In some cases, people infected with the latter pathogen are also at risk for stomach cancer.
For the design of the inhibitors, a detailed study of the phosphoryl-transfer mechanism catalyzed by the enzyme was first performed. To this end, a simple and rapid method of 1H and 31P Nuclear Magnetic Resonance spectroscopy was developed. This approach established, in contrast to the previously reported associative one, that the mechanism is dissociative. Based on the mechanism of action and a detailed computational study of the structural requirements for substrates binding to the active site, as well as the essential conformational changes for the catalytic regeneration, the design of inhibitors was performed. Reasoning that 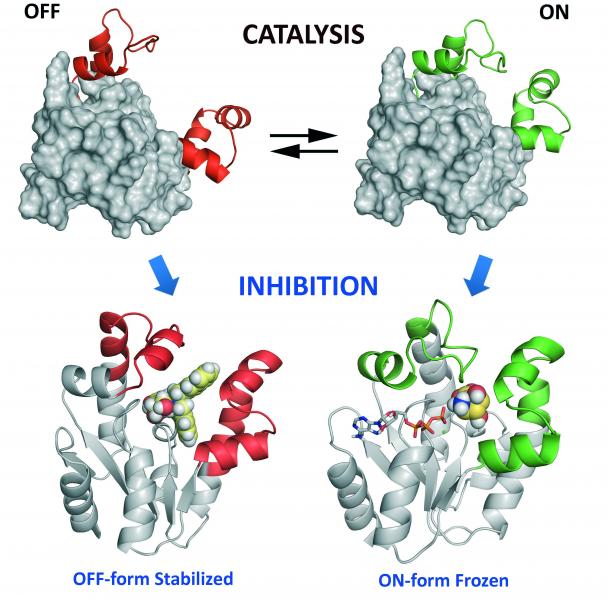 enzymes are "dynamic and flexible" systems able to adopt diverse conformations during catalysis – ranking from the open (inactive, OFF) to the closed form (active, ON), the inhibition of the shikimate kinase was addressed by disabling the enzyme flexibility, which is essential for catalytic regeneration.
enzymes are "dynamic and flexible" systems able to adopt diverse conformations during catalysis – ranking from the open (inactive, OFF) to the closed form (active, ON), the inhibition of the shikimate kinase was addressed by disabling the enzyme flexibility, which is essential for catalytic regeneration.
Diverse compounds capable of stabilizing the active site closed form and others that prevent the closure thereof were synthesized. These investigations led to the identification of several candidates that are very potent against the enzyme having an excellent in vitro activity against M. tuberculosis and H. pylori. Through these studies it was also demonstrated that this motion-based strategy would be an attractive approach for achieving the "selective" inhibition of homologous enzymes with ligands that bind to the same region. Part of the results obtained in this thesis has already been published in four papers in prestigious international journals: J. Am. Chem. Soc. 2013, 135, 12366, Chem. Eur. J. 2016, 22, 2758, J. Med. Chem. 2016, 59, 5471 and Chem. Eur. J. 2016, 22, in press. These results have been also highlighted in the cover of J. Med. Chem. and as a Frontispecie article in Chem. Eur. J.


