Lecture: 'Function of Chirality-Responsive Dynamic Helical Polymers'

Prof. Katsuhiro Maeda (Nano Life Science Institute (WPI-NanoLSI), Kanazawa University)
CiQUS Seminar Room
10:00h
 The helix is an essential structural motif for many biopolymers, as represented by double helix in DNA and α-helix in proteins. Their helical structures are considered to play an important role in performing their excellent functions. Therefore, the synthesis of helical polymers with a controlled helix sense has been attracting much interest in the fields of polymer and supramolecular chemistry not only for mimicking biological helices, but also for developing new chiral materials.
The helix is an essential structural motif for many biopolymers, as represented by double helix in DNA and α-helix in proteins. Their helical structures are considered to play an important role in performing their excellent functions. Therefore, the synthesis of helical polymers with a controlled helix sense has been attracting much interest in the fields of polymer and supramolecular chemistry not only for mimicking biological helices, but also for developing new chiral materials.
In this presentation, I will introduce our recent works on the development of stimuli-responsive helical polymers utilizing structural features of dynamic helical polymers. These polymers showed intriguing chiral discrimination ability and chiral amplification phenomena based on their unique helical conformation [1-3].
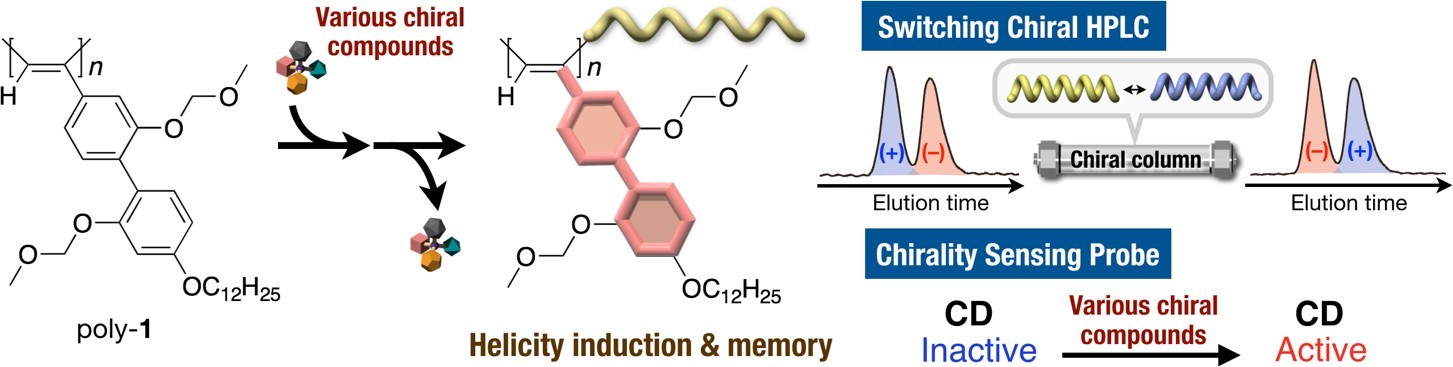
For example, we found that the helix sense of a polyacetylene derivative bearing 2,2’-biphenol-derived pendants (poly-1) was reversibly switched and memorized in the solid state as well as in solution upon interaction with the opposite enantiomeric alcohol followed by its removal, which allowed us to develop the first switchable chiral stationary phase for high performance liquid chromatography (HPLC) [4,5].
Poly-1 also responded to the chirality of a large variety of chiral compounds including alcohols, amines, esters, ethers, amino acid derivatives, and hydrocarbons, thus showing induced circular dichroisms (CDs) in the polymer backbone region due to the formation of preferred-handed helix. Owing to the chiral memory effect, poly-1 can detect the chirality of an extremely small amount of chiral compounds including chiral hydrocarbons [6].
References:
[1] Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K., Chem. Rev. 2009, 109, 6102-6211.
[2] Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K., Chem. Rev. 2016, 116, 13752-13990.
[3] Maeda, K.; Yashima, E., Top. Curr. Chem. 2017, 375, 72.
[4] Shimomura, K.; Ikai, T.; Kanoh, S.; Yashima, E.; Maeda, K., Nat. Chem. 2014, 6 (5), 429-434.
[5] Ishidate, R.; Shimomura, K.; Ikai, T.; Kanoh, S.; Maeda, K., Chem. Lett. 2015, 44, 946-948.
[6] Maeda, K.; Hirose, D.; Okoshi, N.; Shimomura, K.; Wada, Y.; Ikai, T.; Kanoh, S.; Yashima, E., J. Am. Chem. Soc. 2018, DOI: 10.1021/jacs.7b10981.


