Lecture: 'Asymmetric Total Synthesis of Alkaloid Natural Products without External Chiral Sources'

Prof. Sanghee Kim (Seoul National University - Seoul - South Korea)
Aula de Seminarios del CiQUS
12:15h
 The chirality of a starting material having a chiral sp3-carbon can be preserved under some circumstances in the reaction product even though the reaction proceeds at the chiral carbon as a reaction center through reactive intermediates. This process is defined as Memory of chirality (MOC). MOC is an attractive strategy for asymmetric synthesis, but it has found limited applications. There are only few reports of MOC being applied in the total synthesis of natural products.
The chirality of a starting material having a chiral sp3-carbon can be preserved under some circumstances in the reaction product even though the reaction proceeds at the chiral carbon as a reaction center through reactive intermediates. This process is defined as Memory of chirality (MOC). MOC is an attractive strategy for asymmetric synthesis, but it has found limited applications. There are only few reports of MOC being applied in the total synthesis of natural products.
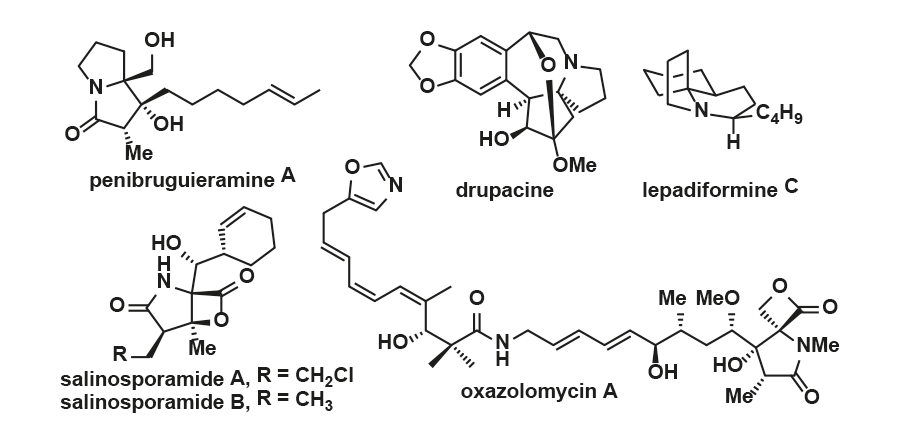
In recent years, we have been involved in the total synthesis of biological interesting alkaloid natural products and their analogues without the aid of external chiral influences. Representative alkaloids of such interest include penibruguieramine, drupacine, lepadiformines, salinosporamides, and oxazolomycins. The principle of MOC is applied for the asymmetric synthesis of these alkaloids using an appropriate amino acid as the only chiral source.
In this presentation, I would like to share our old and new progress on this subject. A mechanistic rationale would be discussed for the excellent stereochemical outcome of MOC reactions.