 Amyloid fibrils are insoluble aggregates with fibrillar morphology and β-sheet-rich structure that cause several diseases called amyloidosis due to their misfolding. Among the different amyloid-like peptides, those called amyloid-β are very important because of their primal role in the Alzheimer’s disease. They accumulate as insoluble plaques at the brain formed mainly by peptides of 40 and 42 aminoacids (Aβ40 and Aβ42), being the latter at higher proportion.
Amyloid fibrils are insoluble aggregates with fibrillar morphology and β-sheet-rich structure that cause several diseases called amyloidosis due to their misfolding. Among the different amyloid-like peptides, those called amyloid-β are very important because of their primal role in the Alzheimer’s disease. They accumulate as insoluble plaques at the brain formed mainly by peptides of 40 and 42 aminoacids (Aβ40 and Aβ42), being the latter at higher proportion.
Applying our experience in detailed photophysical studies and in single molecule techniques we pursue several research lines:
- Study of the formation of early aggregates of amyloids-β as toxic agents in Alzheimer's disease
- Development of fluorescent markers for the quantitative detection of amyloid-β aggregates
- Photophysical characterization of Thioflavin T (ThT), the most common marker used for the detection of amyloid fibrils
Photophysical characterization of Thioflavin T (ThT)
Photophysical study of Thioflavin T as fluorescence marker of amyloid fibrils
Sonia Freire, Marcus H. de Araujo, Wajih Al-Soufi, Mercedes Novo
Dyes and Pigments 2014, 110, 97-105, DOI 10.1016/j.dyepig.2014.05.004
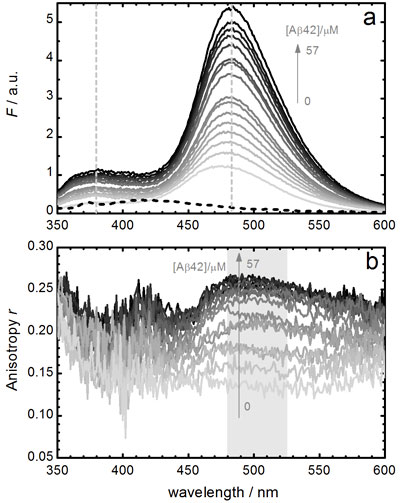
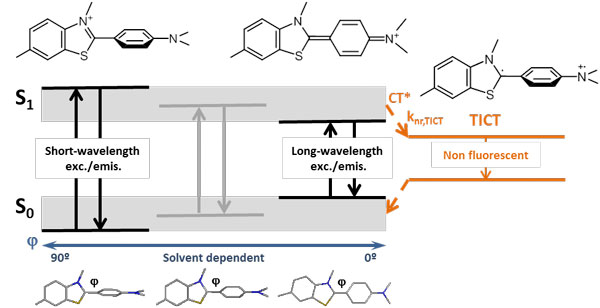 Thioflavin T is a highly sensitive fluorescent marker of amyloid fibrils that has been widely used for in vitro biomedical assays. However, neither its complex photophysical behavior nor its binding mode to amyloid fibrils are still well understood. We present a detailed analysis of the photophysical properties of Thioflavin T in various media, including solvents and solvent mixtures of different viscosities as well as fibrillar and globular proteins. We propose a model that explains the strong wavelength dependency of the Thioflavin T fluorescence and the large fluorescence enhancement in certain environments. We determine the binding affinities and the fluorescence properties of Thioflavin T bound to amyloid-b (1-42) fibrils and to bovine serum albumin and discuss the sensitivity and the specificity of this probe to amyloid aggregates. These results allow us to assess the suitability of Thioflavin T for quantitative determinations in biomedical studies.
Thioflavin T is a highly sensitive fluorescent marker of amyloid fibrils that has been widely used for in vitro biomedical assays. However, neither its complex photophysical behavior nor its binding mode to amyloid fibrils are still well understood. We present a detailed analysis of the photophysical properties of Thioflavin T in various media, including solvents and solvent mixtures of different viscosities as well as fibrillar and globular proteins. We propose a model that explains the strong wavelength dependency of the Thioflavin T fluorescence and the large fluorescence enhancement in certain environments. We determine the binding affinities and the fluorescence properties of Thioflavin T bound to amyloid-b (1-42) fibrils and to bovine serum albumin and discuss the sensitivity and the specificity of this probe to amyloid aggregates. These results allow us to assess the suitability of Thioflavin T for quantitative determinations in biomedical studies.
Fluorescent markers for the quantitative detection of beta-amyloid aggregates
Towards ratiometric sensing of amyloid fibrils in vitro
 Sonia Freire, Flor Rodríguez-Prieto, M. Carmen Ríos Rodríguez, J. Carlos Penedo, Wajih Al-Soufi, and Mercedes Novo
Sonia Freire, Flor Rodríguez-Prieto, M. Carmen Ríos Rodríguez, J. Carlos Penedo, Wajih Al-Soufi, and Mercedes Novo
Chemistry - A European Journal 2015, 21, 3425–3434. DOI: 10.1002/chem.201406110
The aggregation of amyloid-β peptide and its accumulation in the human brain has an important role in the etiology of Alzheimer’s disease. Thioflavin T has been widely used as fluorescent marker of these amyloid aggregates. Nevertheless, its complex photophysical behavior, with strong wavelength dependencies of all its fluorescence properties, requires searching for new fluorescent probes. We propose the use of 2-(2’-hydroxyphenyl)imidazo[4,5-b]pyridine (HPIP), which shows two emission bands and a rich excited-state behavior due to the existence of excited-state intramolecular processes of proton transfer (ESIPT) and charge transfer (TICT). These properties determine a high sensitivity of HPIP fluorescence to its microenvironment and cause a large differential fluorescence enhancement of the two bands upon binding to aggregates of the amyloid-β peptide. Based on this behavior, a very sensitive ratiometric method is established for detection and quantification of amyloid fibrils, which can be combined with the monitoring of fluorescence anisotropy. Binding selectivity of HPIP is discussed on the basis of the apparent binding equilibrium constants of this probe to amyloid-β (1-42) fibrils and to the non-fibrillar protein bovine serum albumin (BSA). Finally, an exhaustive comparison between HPIP and Thioflavin T is presented in order to discuss the sensitivity and specificity of these probes to amyloid aggregates and the significant advantages of the HPIP dye for quantitative determinations.



